Chemical stability and detection methods of hyperforin extract
Understanding the chemical stability and analytical detection of hyperforin extract represents one of the most critical challenges in the development and quality control of botanical pharmaceuticals. This remarkable compound, derived from St. John's wort (Hypericum perforatum), has garnered significant attention from researchers and manufacturers alike due to its unique therapeutic properties and complex analytical requirements. The delicate nature of hyperforin presents both opportunities and challenges for those working in pharmaceutical development, quality assurance, and clinical research.

English name: Hypericum perforatum extract
Latin Name: Hypericum perforatum L.
CAS No.: 548-04-9
Molecular forula:C30H16O8
Molecular Weight: 504.45
Active ingredients: Hypericins, Hyperforin
Specification: 0.3% Hypericins UV; 0.3% Hypericin HPLC; 3%, 98% Hyperforin HPLC
Use Part : Whole herbs
Appearance: Brown powder
Mesh size:80 Mesh
Test Method: HPLC/UV
Chemical Stability
The chemical stability of hyperforin extract presents unique challenges that require careful consideration throughout the entire supply chain, from initial extraction to final product formulation. Hyperforin is notably unstable in the presence of light and oxygen, with pure hyperforin being poorly soluble in water and demonstrating poor stability when exposed to these environmental factors. This instability fundamentally shapes how manufacturers must approach storage, processing, and packaging of hyperforin-containing products.
The degradation mechanisms of hyperforin involve complex oxidative processes that lead to the formation of various breakdown products. Frequent oxidized forms contain a C3 to C9 hemiketal/heterocyclic bridge or will form furan/pyran derivatives. These degradation pathways not only reduce the concentration of active hyperforin but also potentially create compounds with different biological activities, making stability control essential for maintaining consistent therapeutic effects.
Temperature plays a crucial role in hyperforin stability, with research demonstrating that storage conditions significantly impact degradation rates. Studies have shown that hyperforin, hypericin, and pseudohypericin were more stable at -20°C, with decay being lowest at -20°C and highest at 40°C–75% relative humidity. This temperature sensitivity necessitates cold chain management for products containing significant concentrations of hyperforin extract.

Stability Considerations: The instability of hyperforin extract in standard storage conditions requires specialized handling protocols. Manufacturers must implement protective measures including amber glass containers, nitrogen flushing, and temperature-controlled environments to maintain compound integrity.
The solvent environment also influences hyperforin stability significantly. Hyperforin appears to be more stable in ethanol and methanol than as a solid, suggesting that liquid formulations may offer advantages for long-term stability. This characteristic has important implications for extract preparation and formulation strategies, particularly for products intended for extended shelf life.
Light exposure represents another critical stability factor that manufacturers must address. The photosensitive nature of hyperforin requires careful consideration of packaging materials, processing environments, and storage facilities. The instability in the presence of light was more pronounced in extract solutions both for hypericin and pseudohypericin, indicating that light protection becomes even more critical when hyperforin is present in complex extract matrices.
Detection Methods
The analytical detection of hyperforin extract demands sophisticated chromatographic techniques due to the compound's unique chemical properties and potential interference from other botanical constituents. High-Performance Liquid Chromatography (HPLC) has emerged as the gold standard for hyperforin quantification, offering the precision and selectivity required for both quality control and research applications.
Multiple detection approaches have been developed to address the specific analytical challenges associated with hyperforin extract. Reversed-phase HPLC methods with photodiode array detection have been established for the simultaneous analysis of hypericin and hyperforin in methanolic extracts. This approach allows for comprehensive analysis of multiple bioactive compounds within a single analytical run, improving efficiency while maintaining analytical rigor.
Electrochemical detection represents another sophisticated approach to hyperforin analysis that offers distinct advantages in certain applications. HPLC methods with electrochemical detection for hyperforin determination have been developed and validated, allowing hyperforin extracts to be analyzed without additional sample precleaning. This streamlined approach can significantly reduce analysis time and minimize potential sources of analytical error.
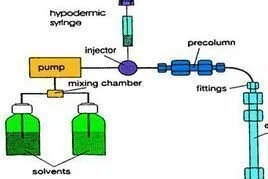
The development of methods for biological sample analysis has expanded the analytical capabilities for hyperforin research. Analysis of hypericin, pseudohypericin, and hyperforin in biological fluids has been reported using single-drop liquid-phase microextraction in conjunction with HPLC-UV-fluorescence detection, with samples separated by isocratic reversed-phase HPLC and analyzed using fluorimetric detection for hypericin/pseudohypericin and UV detection for hyperforin. These methods enable pharmacokinetic studies and bioavailability assessments.

Sample preparation techniques play a critical role in achieving accurate hyperforin detection results. Solid-phase extraction (SPE) and isocratic high-performance liquid chromatography analysis with ultraviolet detection have been developed to determine hyperforin in human plasma samples. The selection of appropriate internal standards, such as benzo[k]fluoranthene, helps ensure analytical precision and accuracy across different sample matrices.
Analytical Precision: Modern detection methods for hyperforin extract combine multiple analytical techniques to overcome the challenges posed by the compound's instability and matrix complexity. These sophisticated approaches ensure reliable quantification for both research and commercial applications.
Rebecca: Hyperforin Extract Manufacturer
The complexities surrounding chemical stability and detection methods of hyperforin underscore the critical importance of working with experienced manufacturers who understand these technical challenges. The sophisticated analytical requirements and stringent stability considerations demand expertise that extends far beyond basic extraction processes. For researchers, formulators, and manufacturers seeking reliable supplies, partnering with knowledgeable providers becomes essential for project success.
Rebecca stands as a trusted hyperforin extract supplier, combining deep technical understanding with rigorous quality control measures to deliver products that meet the demanding requirements of modern pharmaceutical and research applications. Our product (CAS No..: 548-04-9) undergoes comprehensive analytical testing using advanced HPLC/UV methodologies to ensure accurate quantification of active ingredients, including hypericins and hyperforin.
Our commitment to quality extends to every aspect of extract production and handling. We maintain precise specifications, including 0.3% Hypericins UV, 0.3% Hypericin HPLC, and concentrations of 3% and 98% Hyperforin HPLC, all processed to 80 Mesh particle size for optimal consistency. Each batch undergoes rigorous testing using validated HPLC/UV methods that account for the stability challenges and detection complexities discussed throughout this article.
Understanding the critical nature of proper handling and storage, Rebecca implements comprehensive stability protocols throughout our supply chain. From controlled extraction environments to specialized packaging systems, we ensure that the hyperforin you receive maintains its integrity and potency. Our technical team stays current with the latest developments in analytical methods and stability research, continuously improving our processes to deliver superior products.
The analytical expertise required for reliable hyperforin detection demands sophisticated equipment and extensive experience with chromatographic techniques. Rebecca's quality control laboratory utilizes state-of-the-art HPLC systems with multiple detection methods, enabling us to provide comprehensive certificates of analysis that meet regulatory requirements and support your specific application needs.
For researchers and manufacturers who recognize the importance of technical excellence in hyperforin supply, Rebecca offers the reliability and expertise necessary for successful project outcomes. Our commitment to quality, combined with a deep understanding of stability and analytical challenges, makes us the preferred partner for demanding applications requiring the highest standards of product consistency and purity.
For more information about our product specifications, analytical capabilities, stability data, or to place an order, please reach out to us at information@sxrebecca.com. Our technical team is ready to discuss your specific requirements and provide the expert support necessary for your hyperforin applications.
References
Chemical stability data from multiple sources including Wikipedia, ScienceDirect publications, and peer-reviewed stability studies on Hypericum perforatum extracts.
Analytical methods from PubMed indexed studies, ScienceDirect publications, and peer-reviewed chromatographic research on hyperforin detection techniques.








