How is baicalin extracted from Scutellaria baicalensis?
Baicalin extract has evolved significantly from ancient decoction methods to contemporary industrial-scale operations. The fundamental challenge lies in efficiently separating this valuable flavonoid from the complex matrix of plant materials while preserving its biological activity. Modern extraction techniques have addressed many limitations of traditional methods, offering improved yields, reduced processing times, and enhanced purity levels that meet pharmaceutical standards.

English name: Baikal skullcap root extract
Latin Name: Scutellaria Baicalensis Georgi. L .
CAS No.: 21967-41-9
Molecular forula:C21H18O11
Molecular Weight:446.37
Active ingredients: Baicalin
Specification: 70-98%
Use Part : Root
Appearance: Light yellow fine powder
Mesh size:80 Mesh
Test Method: HPLC
Extraction Methods
Traditional extraction methods for baicalin have provided the foundation for modern techniques, with water decoction being the most historically significant approach. This method involves boiling dried Scutellaria baicalensis roots in water for extended periods, typically 2-4 hours, allowing the water-soluble compounds to dissolve into the liquid phase. While this technique has been used for centuries in traditional Chinese medicine, it presents several limitations including low extraction efficiency, high energy consumption, and potential degradation of heat-sensitive compounds.
Heat reflux extraction represents a significant improvement over simple decoction, utilizing controlled temperature and continuous solvent circulation to enhance extraction efficiency. This method typically employs ethanol-water mixtures at concentrations ranging from 50-80% ethanol, with extraction temperatures maintained between 60-80°C for 1-3 hours. The reflux system ensures consistent solvent contact with plant material while preventing solvent loss through evaporation, resulting in higher baicalin powder yields compared to traditional water extraction.
Ultrasonic-assisted extraction has emerged as a highly effective modern technique, utilizing high-frequency sound waves to disrupt cell walls and facilitate the release of intracellular compounds. This method significantly reduces extraction time to 30-60 minutes while maintaining or improving extraction yields. The mechanical effects of ultrasonic waves create cavitation bubbles that enhance mass transfer between the solid plant material and liquid solvent, making this technique particularly valuable for extracting compounds with structures similar to those found in siberian ginseng extract.
Microwave-assisted extraction offers another innovative approach, using microwave energy to heat the solvent and plant material simultaneously. This technique provides rapid heating, reduced extraction times, and improved selectivity for target compounds. The microwave energy causes rapid heating of water molecules within plant cells, creating internal pressure that facilitates cell wall rupture and compound release. This method has shown particular promise for extracting flavonoids like baicalin powder while maintaining their biological activity.
Supercritical fluid extraction represents the most advanced extraction technology currently available, using carbon dioxide under specific pressure and temperature conditions to create a supercritical fluid with properties of both liquid and gas phases. This method offers exceptional selectivity, produces solvent-free extracts, and operates at relatively low temperatures that preserve compound integrity. While requiring significant capital investment, supercritical extraction produces the highest quality baicalin extracts with minimal environmental impact.


Key Considerations for Extraction
Solvent selection plays a crucial role in determining extraction efficiency and final product quality. Water serves as the most traditional solvent, effectively extracting baicalin powder due to its polar nature and ability to form hydrogen bonds with the compound's hydroxyl groups. However, pure water extraction often results in the co-extraction of unwanted compounds such as sugars, proteins, and tannins that complicate purification processes. Ethanol-water mixtures have proven more selective, with optimal concentrations typically ranging from 60-80% ethanol depending on the specific extraction method employed.
Temperature optimization requires balancing extraction efficiency with compound stability. Higher temperatures generally increase extraction rates by enhancing solvent penetration and compound solubility, but excessive heat can lead to baicalin powder degradation or unwanted chemical reactions. Most extraction methods achieve optimal results at temperatures between 60-80°C, though this parameter must be adjusted based on the specific solvent system and extraction technique used. The temperature considerations for baicalin extraction often parallel those required for extracting eleutherosides from Siberian ginseng extract, where similar thermal sensitivity concerns apply.
Particle size and solid-to-liquid ratios significantly impact extraction efficiency by affecting the surface area available for mass transfer. Finely ground plant material (typically 40-80 mesh) provides increased surface area for solvent contact, leading to improved extraction rates. However, overly fine particles can create extraction difficulties due to poor solvent flow and potential clogging of filtration systems. The optimal solid-to-liquid ratio typically ranges from 1:10 to 1:20 (w/v), balancing extraction efficiency with practical processing considerations.


Purification of Baicalin
The purification of baicalin from crude extracts represents a critical step in producing high-quality pharmaceutical or nutraceutical ingredients. Initial purification typically begins with solvent removal through evaporation or concentration, resulting in a crude extract containing baicalin along with other plant compounds. This crude material requires further processing to achieve the purity levels necessary for commercial applications, often targeting baicalin powder of 85-98% depending on intended use.
Liquid-liquid extraction serves as an effective preliminary purification technique, utilizing the differential solubility of baicalin powder in various solvent systems. This method typically employs ethyl acetate or n-butanol to selectively extract baicalin from aqueous solutions, leaving behind more polar impurities such as sugars and proteins. The process can be repeated multiple times to achieve higher purity levels, though care must be taken to optimize conditions that maximize baicalin recovery while minimizing co-extraction of unwanted compounds.
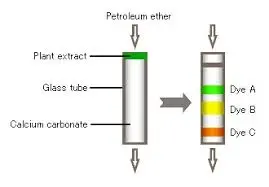
Column chromatography provides more sophisticated purification capabilities, utilizing stationary phases that separate compounds based on their chemical properties. Silica gel chromatography has proven particularly effective for baicalin purification, with mobile phases consisting of chloroform-methanol mixtures in various ratios. This technique allows for fine-tuning of separation conditions to achieve high purity levels while maintaining good recovery rates. The principles used in baicalin purification often apply to other plant compounds, including the separation of eleutherosides in Siberian ginseng extract processing.
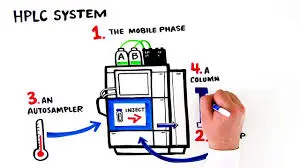
High-performance liquid chromatography (HPLC) represents the gold standard for baicalin powder purification, offering exceptional resolution and purity levels. Preparative HPLC systems can process significant quantities of crude extract while achieving purities exceeding 95%. The technique utilizes reversed-phase columns with acetonitrile-water mobile phases, allowing for precise control of separation conditions. While more expensive than other purification methods, HPLC provides unmatched purity and consistency for high-value applications.
Rebecca: Baicalin Powder For Sale
Looking for high-quality baicalin powder? Rebecca is your reliable supplier. Our product features active ingredients of baicalin with specifications ranging from 70-98%, ensuring a variety of choices based on your needs. The product has a uniform mesh size of 80 Mesh, guaranteeing fine and smooth particles. And we use the HPLC test method to ensure accuracy and reliability. Whether you're in the pharmaceutical, supplement, or research industry, Rebecca can provide you with the premium baicalin you need. We're committed to offering competitive pricing and excellent service. Start your journey to excellent baicalin by reaching out to us at information@sxrebecca.com. Don't miss the opportunity to enhance your formulations with our top-notch product.
References
- Zhang, Q., Zhao, J., Xu, J., Feng, F., & Qu, W. (2015). Medicinal uses, phytochemistry and pharmacology of the genus Scutellaria. Journal of Ethnopharmacology, 175, 475-484.
- Guo, H., Liu, A., Ye, M., Yang, M., & Guo, D. (2007). Characterization of phenolic compounds in the fruits of Forsythia suspensa by high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Rapid Communications in Mass Spectrometry, 21(5), 715-729.
- Li, J., Ding, S., Ding, X., et al. (2009). Optimization of baicalin water extraction process from Scutellaria baicalensis by using orthogonal test and HPLC. Revista Brasileira de Farmacognosia, 19(2B), 568-572.
- Gao, Y., Li, G., Ma, L., et al. (2016). Chemical and pharmacological evaluations on the extract of Scutellaria baicalensis Georgi prepared by various extraction methods. SpringerPlus, 5(1), 1408.
- Xu, H., Shen, L., Xu, J., et al. (2006). Optimization of extraction, separation and purification of baicalin in Scutellaria baicalensis using response surface methodology. Journal of Pharmaceutical and Biomedical Analysis, 41(3), 916-922.








