How to extract thymol from thyme?
Thyme, a fragrant herb from the Lamiaceae family, is renowned for its culinary uses and medicinal properties. One of the most valuable components found in thyme is thymol, a natural monoterpene phenol with remarkable antimicrobial, antioxidant, and anti-inflammatory properties. The extraction of thymol from thyme fruit extract has gained significant attention in the pharmaceutical, food, and cosmetic industries due to its versatile applications. This article explores the various methods used to extract thymol from thyme, focusing on efficiency, yield, and quality of the extract.

Before diving into extraction methods, it's important to understand that thymol is primarily found in the essential oil of thyme, which comprises about 20-54% of the volatile compounds, depending on the thyme variety and growing conditions. The concentration of thymol is particularly noteworthy, as the fruits (technically the dried seeds) contain concentrated essential oils that make them an excellent source for extraction.
Steam Distillation (for Volatile Oil Containing Thymol)
Steam distillation represents one of the oldest and most widely used methods for extracting essential oils from aromatic plants, including thyme. This technique is particularly effective for isolating thymol from thyme fruit extract due to its volatile nature. The process leverages the fact that when steam passes through plant material, it carries volatile compounds that can later be condensed and separated.
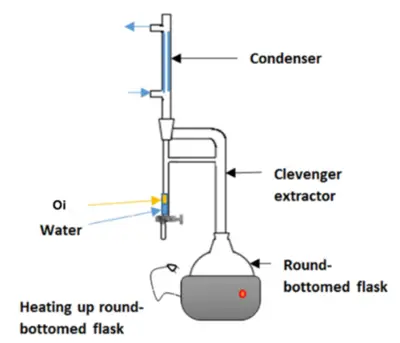
The procedure begins with the preparation of plant material. Fresh or dried thyme fruits are crushed or ground to increase the surface area, which facilitates better extraction of the essential oil. The prepared plant material is then placed in a distillation vessel, and steam is passed through it. As the steam makes contact with the plant material, it causes the cell walls to rupture, releasing the essential oil containing thymol.
The steam, now carrying the volatile compounds, moves into a condenser where it cools and returns to liquid form. This condensate consists of two immiscible layers: water and essential oil. Since the essential oil (including thymol) is less dense than water, it forms the upper layer, which can be easily separated using a separator funnel.
The advantages of steam distillation for thymol extraction include:
1. Purity: The method provides relatively pure essential oil with minimal contamination from non-volatile compounds.
2. Cost-effectiveness: The equipment required is relatively simple and economical to operate, making it suitable for small to medium-scale production.
3. Safety: Unlike some solvent-based methods, steam distillation doesn't involve potentially harmful organic solvents, making it a safer option for both operators and end users.
However, steam distillation also has limitations. The yield of essential oil is typically around 1-2% of the dry weight of thyme, which might be considered low compared to some other extraction methods. Additionally, the process requires significant energy for steam generation, and prolonged exposure to heat may degrade some heat-sensitive compounds in the thyme fruit extract.
To optimize thymol extraction via steam distillation, several parameters can be adjusted, including distillation time, steam flow rate, and particle size of the plant material. Research has shown that extending distillation time beyond 3 hours provides minimal additional yield, and optimal steam flow rates vary depending on the specific equipment used.
Solvent Extraction (for Higher Yield or Non-Volatile Matrixes)
Solvent extraction offers an alternative approach to isolating thymol from thyme fruit extract, particularly when higher yields are desired or when dealing with non-volatile matrices. This method utilizes the principle of like dissolves like, employing organic solvents to dissolve thymol and other desired compounds from the plant material.
Several solvents can be used for thymol extraction, including ethanol, methanol, hexane, and acetone. Each solvent offers different selectivity and extraction efficiency. Ethanol is often preferred due to its relatively low toxicity and good extractability for phenolic compounds like thymol.
The solvent extraction process typically involves the following steps:
1. Preparation: The thyme fruits are dried and ground to increase surface area for better solvent penetration.
2. Extraction: The plant material is mixed with the selected solvent and agitated to facilitate the dissolution of thymol and other target compounds into the solvent. This can be performed at room temperature or under mild heating to improve extraction efficiency.
3. Filtration: The mixture is filtered to separate the solid plant material from the liquid extract.
4. Solvent removal: The solvent is evaporated under reduced pressure using a rotary evaporator, leaving behind a concentrated extract containing thymol.
5. Purification: Further purification steps, such as chromatograph,y may be employed to isolate thymol from other extracted compounds.
Modern variations of solvent extraction include:
The advantages of solvent extraction include higher yields compared to steam distillation (typically 3-7% extract by dry weight), the ability to extract both volatile and non-volatile compounds, and flexibility in terms of selectivity based on solvent choice. However, the method also presents challenges, including potential solvent residues in the final product, environmental concerns regarding solvent disposal, and higher operational costs compared to steam distillation.

Supercritical Fluid Extraction (SFE, Industrial/High-Purity)
Supercritical Fluid Extraction (SFE) represents the cutting edge of extraction technologies for obtaining high-purity thymol from thyme fruit extract. This method utilizes the unique properties of supercritical fluids, substances that exist above their critical temperature and pressure points, exhibiting characteristics of both liquids and gases. Carbon dioxide (CO2) is the most commonly used supercritical fluid due to its moderate critical parameters (31.1°C, 73.8 bar), non-toxicity, non-flammability, and ability to be easily removed from the final product.
The SFE process for extracting thymol from thyme involves several key steps:
1. Sample preparation: Dried thyme fruits are ground to an optimal particle size to facilitate efficient extraction.
2. System pressurization: CO2 is compressed and heated beyond its critical point to create the supercritical fluid.
3. Extraction: The supercritical CO2 passes through an extraction vessel containing the prepared thyme material. Under these conditions, the fluid exhibits excellent solvating power for non-polar compounds like thymol.
4. Separation: After extraction, the pressure is reduced, causing the CO2 to return to its gaseous state and separate from the extracted compounds, which precipitate in a collection vessel.
5. Collection: The extracted thymol and other compounds are collected, while the CO2 can be recycled back into the system.
One of the most significant advantages of SFE for thymol extraction is the ability to fine-tune the selectivity by adjusting operating parameters. For instance, by modifying the pressure (typically between 100-400 bar) and temperature (usually 40-60°C), the density and solvent power of supercritical CO2 can be precisely controlled to target specific compounds. This selectivity allows for the extraction of thymol from thyme fruit extract with minimal co-extraction of unwanted substances.
Research has shown that SFE can achieve thymol yields of up to 90% of the theoretically extractable amount from thyme, making it particularly valuable for high-value applications where purity and quality are paramount. The technique has been successfully scaled up to industrial levels, with commercial production of high-purity thyme fruit extract for pharmaceutical and food applications.

Rebecca: Thyme Fruit Extract
The extraction of thymol from thyme represents a fascinating intersection of traditional knowledge and modern technology. From the time-tested method of steam distillation to the cutting-edge supercritical fluid extraction, each technique offers unique advantages depending on the intended application, scale of production, and desired purity of the final product. The choice of extraction method significantly impacts the quality, composition, and biological activity of the resulting thyme extract.
As research continues to advance, new hybrid and green extraction technologies are emerging, promising even more efficient and sustainable ways to harness the therapeutic potential of thymol from thyme. These developments will undoubtedly contribute to the growing market for natural plant-based products and expand the applications of thymol in various industries.
Rebecca is a thyme fruit extract supplier,
Specifications: 5:1 10:1 20:1, thymol 5%-10%
Testing Method: TLC
Active ingredients: Thymol
For more information or to place an order, please reach out to us at information@sxrebecca.com.
References
1. Fachini-Queiroz, F.C., et al. (2012). Effects of thymol and carvacrol, constituents of Thymus vulgaris L. essential oil, on the inflammatory response. Evidence-Based Complementary and Alternative Medicine, 2012.
2. Rota, M.C., et al. (2008). Chemical composition and antimicrobial activity of Thymus vulgaris L. essential oil from Spain. Journal of Essential Oil Research, 20(2), 167-174.
3. Chemat, F., et al. (2017). Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrasonics Sonochemistry, 34, 540-560.
4. Fornari, T., et al. (2012). Isolation of essential oil from different plants and herbs by supercritical fluid extraction. Journal of Chromatography A, 1250, 34-48.
5. Reverchon, E., & De Marco, I. (2006). Supercritical fluid extraction and fractionation of natural matter. The Journal of Supercritical Fluids, 38(2), 146-166.








