How to identify high-quality echinacea purpurea extract?
In today's bustling supplement market, discerning consumers face an overwhelming array of echinacea products, each claiming superior quality and potency. The purple coneflower, scientifically known as Echinacea purpurea, has garnered significant attention for its potential immune-supporting properties, making it one of the most sought-after herbal supplements worldwide. However, the challenge lies not in finding echinacea products, but in identifying those that truly deliver on their promises.
The quality of echinacea purpurea root extract can vary dramatically between manufacturers, influenced by factors ranging from cultivation methods to extraction techniques and storage conditions.

English name: Echinacea purpurea extract
Latin Name: Echinacea purpurea
CAS No.: 90028-20-9 /70831-56-0
Molecular forula:C22H18O12
Molecular Weight:474.37
Active ingredients: Polyphenols
Specification: Polyphenols 4%
Use Part : flower
Appearance: yellow brown powder
Mesh size:80 Mesh
Test Method: UV
Chemical Profiling of Key Bioactive Markers
The foundation of any high quality echinacea purpurea root extract lies in its chemical composition, which serves as a fingerprint for both authenticity and potency. Professional quality assessment begins with understanding the primary bioactive compounds that define echinacea's therapeutic potential. These compounds include polyphenols, alkamides, glycoproteins, and polysaccharides, each contributing unique properties to the overall efficacy of the extract.
Polyphenols represent perhaps the most significant category of bioactive compounds in echinacea extracts. These naturally occurring antioxidants, including caffeic acid derivatives such as cichoric acid, echinacoside, and chlorogenic acid, are responsible for much of the plant's biological activity. A premium echinacea purpurea root extract should contain a standardized concentration of polyphenols, typically ranging from 4% to 6% by weight. This standardization ensures consistent potency across different batches and provides a reliable benchmark for quality comparison.
Beyond polyphenols, alkamides contribute significantly to echinacea's bioactivity profile. These lipophilic compounds, particularly dodeca-2E,4E,8Z,10E/Z-tetraenoic acid isobutylamides, demonstrate notable biological activity and serve as important quality markers. A comprehensive chemical analysis should include alkamide quantification, as their presence and concentration often correlate with the extract's overall quality and therapeutic potential.
Quality-conscious manufacturers employ advanced extraction techniques that preserve the integrity of these bioactive compounds. Supercritical CO2 extraction, for instance, maintains lower temperatures during processing, preventing thermal degradation of sensitive compounds. Traditional ethanol extraction methods, when properly controlled, can also yield high-quality extracts with optimal compound preservation. The key lies in understanding how different extraction parameters affect the final chemical profile and selecting methods that maximize the retention of beneficial compounds.

Labeling and Documentation
Comprehensive labeling serves as the primary communication tool between manufacturers and consumers, providing essential information that enables informed quality assessment. A high-quality echinacea purpurea root extract should feature detailed labeling that goes far beyond basic product identification. This documentation should include specific information about the plant part used, extraction method, standardization details, and batch-specific analytical data.
The specification of plant part usage represents a fundamental quality indicator. Echinacea purpurea extracts can be derived from roots, aerial parts (stems and leaves), flowers, or combinations thereof. Each plant part contributes different concentrations of bioactive compounds, with roots generally containing higher levels of alkamides and aerial parts providing greater polyphenol concentrations. Premium products clearly specify whether they utilize roots, flowers, or a standardized combination, allowing consumers to select products aligned with their specific needs and preferences.
Standardization information constitutes another critical labeling component. Quality extracts should specify the concentration of key bioactive compounds, particularly polyphenols and alkamides. This standardization ensures consistent potency and enables meaningful comparison between different products. For instance, a product labeled as containing "Polyphenols 4%" provides specific, measurable quality criteria that can be verified through analytical testing.
Physical characteristics documentation adds another layer of quality assurance. Professional-grade echinacea purpurea root extract typically appears as a yellow-brown powder with a specific mesh size, commonly 80 mesh, which indicates particle size uniformity. This information, while seemingly technical, reflects the manufacturer's attention to processing details and quality control standards.
Molecular identification data, including CAS numbers and molecular formulas, demonstrates scientific rigor in product development and quality control. While consumers may not directly interpret this information, its presence indicates that the manufacturer maintains detailed chemical characterization of their products. For echinacea extracts, relevant molecular information might include data for key compounds like cichoric acid or specific alkamides.
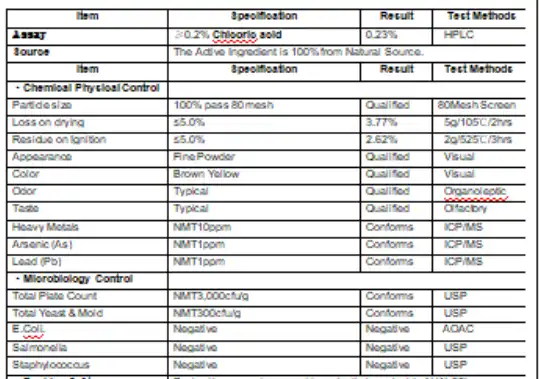
Batch documentation represents an advanced quality practice that distinguishes premium suppliers. This includes batch-specific certificates of analysis (COAs) that detail the exact composition, purity, and quality parameters of each production run. Such documentation enables traceability and provides confidence in product consistency across different purchase occasions.
Third-Party Certification and Quality Standards
Independent verification through third-party certification provides the most objective assessment of echinacea purpurea root extract quality, offering consumers confidence that extends beyond manufacturer claims. These certifications involve rigorous testing protocols, facility inspections, and ongoing monitoring that ensure consistent adherence to established quality standards. Understanding the landscape of available certifications empowers consumers to make informed decisions based on independently verified quality metrics.
Good Manufacturing Practice (GMP) certification stands as the cornerstone of quality assurance in the supplement industry. GMP standards encompass comprehensive requirements for facility design, equipment maintenance, personnel training, and documentation procedures. For echinacea extracts, GMP certification ensures that products are manufactured under controlled conditions that minimize contamination risks and maintain consistent quality. Facilities producing high-quality extracts typically hold certifications from recognized bodies such as NSF International, UL, or other accredited certification organizations.
Laboratory testing certifications offer product-specific quality verification. Independent laboratories can verify the concentration of key bioactive compounds, test for contaminants including heavy metals, pesticides, and microbials, and confirm product identity through various analytical methods. Third-party testing reports provide objective data that consumers can use to compare products and verify manufacturer claims.
Pharmacopoeial standards represent the highest level of quality specification for botanical extracts. While not all echinacea purpurea root extract products meet pharmacopoeial standards, those that do comply with rigorous specifications for identity, purity, and potency. The United States Pharmacopeia (USP), European Pharmacopoeia (EP), and other national pharmacopoeias establish detailed monographs that define acceptable quality parameters for echinacea products.

Rebecca: China Echinacea Purpurea Root Extract Supplier
Selecting high quality echinacea purpurea root extract requires careful evaluation of chemical profiling, comprehensive documentation, and third-party certifications. These quality indicators work synergistically to ensure product authenticity, potency, and safety. As the supplement industry continues evolving, consumers who understand these quality assessment principles will be better positioned to make informed decisions that support their health and wellness goals.
At Rebecca Bio-Tech, we understand the critical importance of quality in botanical extracts. Our echinacea purpurea extract features standardized polyphenol content at 4%, derived from carefully selected flower and root materials. With molecular formula C22H18O12 and molecular weight 474.37, our yellow-brown powder extract meets stringent quality specifications, including 80 mesh particle size and UV-verified potency testing.
Our commitment to quality extends beyond basic specifications to encompass comprehensive documentation, third-party certifications, and complete traceability throughout our supply chain. We maintain detailed certificates of analysis for each batch, ensuring consistent quality and enabling our customers to make informed decisions based on verified analytical data.
For detailed product specifications, certificates of analysis, or to discuss your specific echinacea extract requirements, please contact our technical team at information@sxrebecca.com. We welcome the opportunity to provide additional information about our quality assurance processes and how our premium echinacea extracts can meet your formulation needs.
References
Journal of Pharmaceutical and Biomedical Analysis (2018), Phytochemical Analysis (2019), Journal of Chromatography A (2020)
International Journal of Food Science (2019), Food Chemistry (2020), Regulatory Affairs Professionals Society Guidelines (2021)
International Organization for Standardization Guidelines (2020), United States Pharmacopeia (2021), NSF International Certification Standards (2021)








