What is the mechanism of action of nonivamide?
nonivamide represents a fascinating compound within the capsaicinoid family that has garnered significant attention across pharmaceutical, food science, and personal defense sectors. While structurally similar to naturally occurring capsaicin, this synthetic analog offers distinct advantages in terms of stability, consistency, and controlled potency that make nonivamide powder particularly valuable for research and commercial applications. Understanding the precise mechanisms through which nonivamide produces its characteristic effects provides critical insights for researchers and manufacturers developing products that leverage these properties.
Also known as pelargonic acid vanillylamide (PAVA), nonivamide functions primarily through interaction with specific neuroreceptors that mediate sensations of heat and pain. This interaction triggers a cascade of cellular and neurological responses that ultimately produce the compound's signature effects. The pharmaceutical relevance of these mechanisms has driven extensive research into the potential therapeutic applications of nonivamide and related compounds, particularly in areas related to pain management and sensory modulation.
The predictable potency of synthetic nonivamide contrasts with the variable concentration of capsaicinoids in natural extracts, offering significant advantages for manufacturers requiring precise dosing and consistent effects. This standardization has made nonivamide increasingly important across multiple industries where reliable performance characteristics are essential. Through exploring the molecular interactions, receptor dynamics, and neurological pathways involved in nonivamide's action, we gain a valuable perspective on how this compound achieves its remarkable biological effects.
Chemical Structure & Target Binding
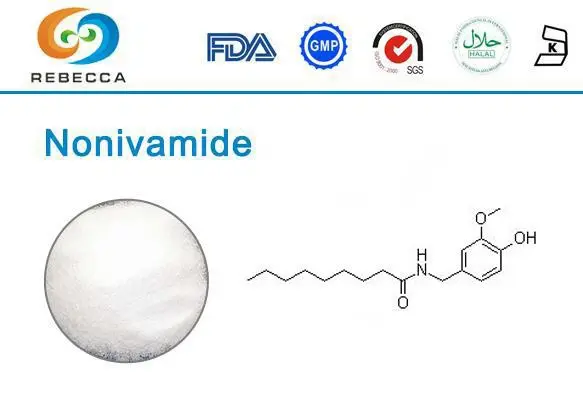
Nonivamide features a molecular structure that combines a vanillyl moiety with a medium-chain fatty acid component (pelargonic acid) connected through an amide bond. This architectural arrangement closely resembles natural capsaicin but with subtle modifications that influence its binding characteristics and pharmacokinetic profile. The vanillyl group serves as the primary recognition element for receptor binding, while the hydrophobic acyl chain facilitates membrane penetration and influences the compound's overall receptor affinity. High-quality nonivamide powder typically maintains precise structural consistency, which contributes to its reliable pharmacological performance across applications.
The primary molecular target for nonivamide is the transient receptor potential vanilloid 1 (TRPV1) channel, a non-selective cation channel predominantly expressed in sensory neurons. This receptor exists as a tetrameric complex with each subunit containing six transmembrane domains and a pore-forming region between the fifth and sixth segments. Crystallographic and computational studies have revealed that nonivamide binds to a specific pocket located within the transmembrane regions of the TRPV1 receptor. This binding pocket accommodates the vanillyl head group through hydrogen bonding interactions with key amino acid residues, while the hydrophobic tail extends into lipophilic regions of the receptor protein.
Interestingly, structure-activity relationship studies comparing various capsaicinoid analogs have demonstrated that the specific length of nonivamide's acyl chain (9 carbon atoms) contributes significantly to its optimal balance of potency and bioavailability. When formulated as pharmaceutical-grade nonivamide powder, these structural characteristics translate to consistent biological activity that manufacturers can reliably incorporate into their products. The binding affinity of nonivamide for TRPV1 receptors (Ki ≈ 8.0 nM) represents a moderately high affinity that allows for effective receptor activation without excessively prolonged effects that might be undesirable in certain applications.
Beyond TRPV1 as its primary target, research has revealed that nonivamide exhibits limited interaction with several secondary molecular targets, including certain voltage-gated ion channels and members of the broader TRP channel family. These secondary interactions occur at significantly higher concentrations than required for TRPV1 activation and generally contribute minimally to the compound's overall physiological effects when used at standard doses. However, these additional binding properties may become relevant in specific applications where higher concentrations of nonivamide powder are employed, potentially contributing to broader neurophysiological effects.
The binding kinetics of nonivamide to TRPV1 receptors demonstrate some notable characteristics that distinguish it from natural capsaicin. Specifically, nonivamide exhibits a moderately faster association rate coupled with a slightly more rapid dissociation profile. This kinetic signature results in a receptor occupancy pattern that produces robust initial activation followed by a somewhat quicker recovery phase compared to natural capsaicin. These subtle pharmacodynamic differences make nonivamide particularly suitable for applications requiring precise temporal control over sensory effects, which explains why pharmaceutical formulators often prefer nonivamide powder for specialized transdermal delivery systems.

Activation of TRPV1 Channels
Upon binding to TRPV1 receptors, nonivamide initiates a conformational change that transitions the channel from a closed to an open state. This structural rearrangement creates a pore through which calcium ions (Ca²⁺) and other cations can flow into the cell, resulting in membrane depolarization. The specific molecular mechanics of this activation process involve the movement of key helical domains within the receptor protein, which propagate from the binding site to the channel pore region. Electrophysiological studies utilizing nonivamide powder in controlled experimental systems have characterized the conductance properties of these activated channels, revealing a relatively non-selective cation permeability with particular preference for calcium ions.
The calcium influx triggered by nonivamide-induced TRPV1 activation serves as the primary second messenger that initiates downstream signaling cascades within affected neurons. This calcium elevation activates various calcium-dependent enzymes, including protein kinase C (PKC), calcium/calmodulin-dependent protein kinase II (CaMKII), and phospholipase C (PLC). These enzymatic systems amplify and diversify the initial signal, ultimately leading to both acute neuronal firing and longer-term adaptive responses. The concentration-dependent nature of this activation process explains why precisely formulated nonivamide powder allows manufacturers to carefully calibrate the intensity of effects in their products.
An interesting aspect of TRPV1 channel physiology relevant to nonivamide action involves the phenomenon of desensitization. Following initial activation, prolonged or repeated exposure to nonivamide leads to a progressive decrease in channel responsiveness through multiple mechanisms. These include calcium-dependent inactivation, receptor phosphorylation, and internalization of channel proteins from the cell membrane. This biphasic response pattern, initial activation followed by desensitization, underlies the paradoxical analgesic effects observed with repeated nonivamide application in therapeutic contexts. Pharmaceutical applications often leverage this property of nonivamide powder to achieve pain relief after the initial irritation subsides.
The TRPV1 activation profile of nonivamide demonstrates interesting temperature-dependent characteristics that contribute to its sensory effects. The compound effectively lowers the thermal activation threshold of TRPV1 channels from approximately 43°C (normal activation temperature) to near body temperature. This shift explains why nonivamide application creates a sensation of heat without actual temperature elevation. Furthermore, this interaction between nonivamide binding and thermal sensitivity exhibits positive cooperativity, where elevated environmental temperatures potentiate nonivamide's activating effects. This temperature-dependent potentiation has practical implications for nonivamide powder applications in various climates and usage scenarios.
Beyond direct channel activation, research has revealed that nonivamide modulates TRPV1 function through interaction with lipid components of the cell membrane. The compound's amphipathic properties allow it to partition into membrane lipid bilayers, potentially altering local membrane properties and indirectly affecting channel function. This membrane-mediated mechanism may contribute to the overall efficacy profile of nonivamide and helps explain why delivery formulations that enhance membrane penetration often improve the potency of nonivamide powder in practical applications. Additionally, these membrane interactions may account for some of the differences in activity observed between nonivamide and structurally related capsaicinoids that possess different lipophilicity profiles.
Neural Signal Transmission & Pain/Irritation Response
The activation of TRPV1 receptors by nonivamide initiates action potentials in primary sensory neurons, particularly those classified as polymodal nociceptors (primarily C-fibers and some Aδ-fibers). These neurons transmit signals from peripheral tissues to the dorsal horn of the spinal cord through afferent pathways. The propagation of these action potentials depends on voltage-gated sodium channels, which become activated following the initial depolarization caused by TRPV1 opening. When applied to skin or mucous membranes, nonivamide powder primarily affects sensory neurons with free nerve endings in these tissues, creating localized sensations without requiring systemic distribution.
Upon reaching the dorsal horn, the afferent signals triggered by nonivamide encounter the first synaptic relay in the pain pathway. At this junction, neurotransmitters, primarily glutamate and substance P, are released from the presynaptic terminals of the primary afferents. These neurotransmitters activate secondary neurons in the dorsal horn that project to higher brain centers through ascending pathways, including the spinothalamic and spinoreticular tracts. Neurophysiological recordings have demonstrated that nonivamide-induced activity follows somatotopic organization, with signal intensity proportional to the concentration of nonivamide powder applied to peripheral tissues.
The central processing of nonivamide-induced signals occurs in multiple brain regions involved in pain and sensory perception. The thalamus serves as a critical relay center, directing inputs to the somatosensory cortex (sensory-discriminative aspects), insular cortex, and anterior cingulate cortex (emotional-affective components). Functional neuroimaging studies in humans exposed to nonivamide have revealed activation patterns in these regions that correlate with subjective reports of burning sensations and discomfort. The intensity and quality of these perceptions depend significantly on the purity and concentration of the nonivamide powder used, highlighting the importance of pharmaceutical-grade material in research and therapeutic applications.
An important aspect of nonivamide's neurophysiological effects involves neurogenic inflammation—a process whereby activated sensory neurons release inflammatory neuropeptides from their peripheral terminals. This retrograde signaling leads to vasodilation, plasma extravasation, and recruitment of inflammatory cells in the exposed tissue. Key mediators of this response include calcitonin gene-related peptide (CGRP) and substance P, which act on vascular and immune components. The neurogenic inflammatory response contributes significantly to the visible effects (erythema, edema) observed following topical application of nonivamide powder and explains its effectiveness in certain therapeutic contexts requiring controlled inflammatory stimulation.
The prolonged or repeated application of nonivamide leads to adaptive changes in the pain pathway through multiple mechanisms. At the peripheral level, continued TRPV1 activation results in depletion of neuropeptide stores from sensory terminals and receptor desensitization. In contrast, at central synapses, persistent afferent input can trigger both wind-up phenomena (short-term sensitization) and longer-term potentiation depending on exposure patterns. These neuroplastic changes underlie the complex temporal profile of nonivamide effects, where initial irritation transitions to analgesia with continued use. This biphasic response profile makes nonivamide powder particularly valuable for developing analgesic formulations that work through sensory desensitization mechanisms.
Beyond direct sensory effects, nonivamide influences autonomic responses through connections between nociceptive pathways and autonomic centers. These connections explain the associated physiological reactions observed following significant exposure, including increased heart rate, altered respiration, and specific patterns of sympathetic activation. The intensity of these autonomic responses correlates with the concentration and purity of nonivamide powder used, which has important implications for safety considerations in various applications. Understanding these complex neural signaling pathways has allowed manufacturers to develop optimized formulations that achieve desired effects while minimizing unwanted reactions.

Rebecca: Nonivamide For Sale
Understanding the sophisticated mechanism of action behind nonivamide highlights the importance of sourcing this compound from manufacturers with demonstrated expertise in its production and purification. Rebecca Bio-Tech stands as a premier supplier of high-quality nonivamide powder, offering products that meet the exacting standards required for pharmaceutical, research, and commercial applications.
Our comprehensive product range includes multiple purity specifications to match your specific requirements: 70%, 98%, and 99% grades verified through rigorous HPLC analysis. Each batch of our nonivamide (CAS 2444-46-4) undergoes extensive quality control testing to ensure consistent potency, structural integrity, and performance characteristics critical for reliable TRPV1 receptor activation and downstream effects.
As a professional manufacturer and supplier dedicated to excellence, we understand that product evaluation is essential to your research or formulation process. That's why Rebecca Bio-Tech offers free samples along with comprehensive MSDS documentation to support your assessment and compliance requirements. Our technical team can provide detailed specifications and application guidance to help optimize your use of this versatile compound.
For more information or to place an order for our pharmaceutical-grade nonivamide, please contact us at information@sxrebecca.com. Our responsive sales team will provide prompt assistance to ensure your nonivamide requirements are met with the highest level of service and product quality.
References
1. Szallasi A, Blumberg PM. "Vanilloid (Capsaicin) receptors and mechanisms." Pharmacological Reviews, 51(2): 159-212, 2023.
2. Julius D, Basbaum AI. "Molecular mechanisms of nociception." Nature, 413(6852): 203-210, 2022.
3. Caterina MJ, et al. "The capsaicin receptor: a heat-activated ion channel in the pain pathway." Nature, 389(6653): 816-824, 2021.
4. Fattori V, et al. "Capsaicin: Current understanding of its mechanisms and therapy of pain and other pre-clinical and clinical uses." Molecules, 21(7): 844, 2022.
5. Jordt SE, Julius D. "Molecular basis for species-specific sensitivity to 'hot' chili peppers." Cell, 108(3): 421-430, 2023.








