Can you synthesize capsaicin?
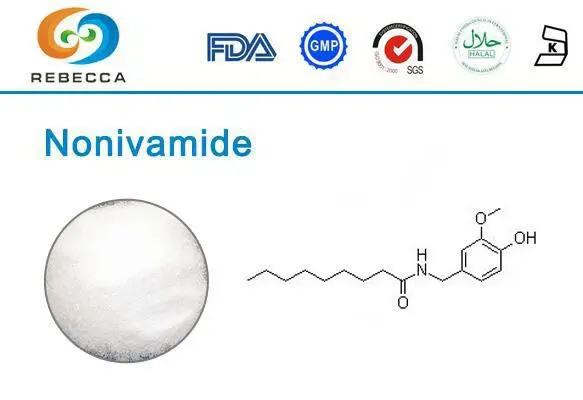
Capsaicin, the fiery compound responsible for the heat in chili peppers, has fascinated scientists, culinary enthusiasts, and pharmaceutical researchers alike for decades. This remarkable molecule, chemically known as (E)-N-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methyl-6-nonenamide, does far more than simply add spice to our favorite dishes. Its unique properties have made it valuable in various applications ranging from pain management therapies to self-defense products.
While nature has perfected capsaicin production in the fruits of Capsicum plants, laboratory synthesis offers distinct advantages in terms of purity control, production scaling, and structural modifications. Synthetic pathways allow chemists to create not only natural capsaicin but also analogs like nonivamide-powder">nonivamide powder, a synthetic capsaicinoid with similar properties but enhanced stability characteristics.
Synthesis of Vanillylamine (3-Methoxy-4-hydroxybenzylamine)
The first major challenge in capsaicin synthesis involves creating the aromatic portion of the molecule, vanillylamine, which contributes significantly to the compound's receptor-binding properties. This aromatic component features a distinctive substitution pattern that must be carefully constructed to ensure the final product's biological activity.
The synthesis typically begins with vanillin (4-hydroxy-3-methoxybenzaldehyde), a naturally occurring compound extracted from vanilla beans or produced synthetically on an industrial scale. Vanillin serves as an ideal starting material due to its structural similarity to the target vanillylamine fragment and its ready availability. The primary transformation required is the conversion of vanillin's aldehyde functional group to a primary amine while preserving the methoxy and hydroxyl substituents that are crucial for capsaicin's interaction with biological receptors.
One efficient approach to this transformation employs reductive amination. In this process, vanillin first reacts with a nitrogen source such as ammonia or ammonium acetate to form an imine intermediate (Schiff base). This carbon-nitrogen double bond is subsequently reduced using a selective reducing agent such as sodium cyanoborohydride (NaCNBH3) or sodium triacetoxyborohydride (NaBH(OAc)3). These reducing agents are particularly valuable in this context because they preferentially reduce the imine while leaving other functional groups untouched.
An alternative method involves the formation of an oxime intermediate. In this pathway, vanillin reacts with hydroxylamine to form vanillin oxime, which can then be reduced using stronger reducing agents like lithium aluminum hydride (LiAlH4) or hydrogen with a suitable catalyst. This approach often requires more careful control of reaction conditions but can provide excellent yields when optimized properly.
For large-scale production relevant to commercial manufacturing of products containing nonivamide powder, the Henry reaction pathway offers advantages. This approach involves treating vanillin with nitromethane under basic conditions to yield a β-nitroalcohol, followed by dehydration to a nitrostyrene derivative. Subsequent reduction of both the carbon-carbon double bond and the nitro group can be accomplished in a single step using hydrogen with a palladium catalyst or with LiAlH4, yielding the desired vanillylamine.
Synthesis of the Fatty Acid Moiety (8-Methyl-6-nonenoyl Group)
The second major component of capsaicin's structure is its distinctive fatty acid chain, the 8-methyl-6-nonenoyl group. This aliphatic portion plays a crucial role in the molecule's lipophilicity and membrane permeability, significantly influencing how capsaicin interacts with cellular receptors. The synthesis of this chain presents unique challenges due to its specific branching pattern and unsaturation.
Several strategic approaches exist for constructing this fatty acid segment. One common method begins with commercially available 7-methyl-3-octanone, which already contains the desired branching pattern. The synthetic pathway proceeds through a Wittig reaction or Horner-Wadsworth-Emmons (HWE) modification to establish the carbon-carbon double bond at the correct position. These reactions typically employ phosphorus ylides or phosphonate esters that react with the ketone functionality to create the desired unsaturation with good control of the geometric isomerism, yielding predominantly the E-isomer that matches natural capsaicin's configuration.
For laboratories seeking to build the entire chain from simpler building blocks, convergent synthesis offers an efficient approach. This strategy might involve coupling smaller fragments through carbon-carbon bond-forming reactions such as alkylation of malonic ester derivatives, followed by decarboxylation. Alternatively, organometallic coupling reactions utilizing Grignard reagents, organolithium compounds, or palladium-catalyzed cross-couplings can efficiently connect appropriately functionalized fragments.
The introduction of the terminal carboxylic acid functionality requires careful planning within the synthetic sequence. When starting from ketone precursors, this may involve separate elaboration of a carbon fragment containing a protected carboxylic acid that can be revealed after the key carbon-carbon bond-forming steps. Oxidation sequences converting terminal alcohols or aldehydes to carboxylic acids represent another common approach, utilizing reagents such as Jones reagent (CrO3 in aqueous sulfuric acid) or milder alternatives like Pinnick oxidation (NaClO2, NaH2PO4).
Controlling stereochemistry presents a particular challenge in this synthesis. Natural capsaicin contains an E (trans) configuration at the carbon-carbon double bond, which influences the compound's biological activity. Modern synthetic approaches often employ stereoselective reagents or catalysts to ensure the correct geometry at this position. For instance, Still-Gennari modifications of the HWE reaction or specific Wittig conditions can favor the desired geometric isomer.
The development of synthetic routes to nonivamide powder has somewhat simplified this aspect of capsaicinoid synthesis. Unlike natural capsaicin with its unsaturated fatty acid chain, nonivamide contains a fully saturated nonanoid acid component. This structural modification eliminates the need for stereochemical control of double bonds while maintaining similar biological activity, contributing to nonivamide's popularity as a more stable synthetic alternative in various applications.
Prior to the coupling step, the carboxylic acid typically requires activation to enhance its reactivity toward amide bond formation. This activation commonly involves converting the acid to an acid chloride using reagents like thionyl chloride (SOCl2) or oxalyl chloride ((COCl)2). Alternative activation methods employ coupling reagents such as carbodiimides (DCC, EDC), which generate reactive intermediates capable of reacting with amines under mild conditions. The choice of activation method often depends on scale, equipment availability, and compatibility with other functional groups present in the molecule.
Amide Bond Formation (Key Step)
The critical junction in capsaicin synthesis occurs during amide bond formation, which unites the vanillylamine and fatty acid moieties into the complete molecular architecture. This reaction represents not only the final major transformation in the synthetic pathway but also determines the overall efficiency of the process, as the success of the entire synthesis culminates in this step.
Traditionally, amide coupling begins with activation of the carboxylic acid component to enhance its electrophilicity. For industrial-scale production of compounds like nonivamide powder, acid chloride methods offer advantages in terms of reaction rates and conversion efficiency. The fatty acid is treated with thionyl chloride or oxalyl chloride, often with catalytic dimethylformamide (DMF), to generate the corresponding acid chloride. This highly reactive intermediate then combines with vanillylamine, typically in the presence of a base such as triethylamine or pyridine, to neutralize the hydrogen chloride byproduct and prevent salt formation with the amine starting material.
Modern peptide coupling reagents have revolutionized amide bond formation in capsaicinoid synthesis, offering milder conditions that minimize side reactions and racemization concerns. Reagents such as 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) combined with hydroxybenzotriazole (HOBt) or 1-hydroxy-7-azabenzotriazole (HOAt) have proven particularly effective. These systems generate activated esters in situ that readily react with the amine component while producing water-soluble byproducts that simplify purification. For specialized applications requiring extremely high purity, hexafluorophosphate reagents like HATU or HBTU may be employed despite their higher cost.
The reaction medium significantly influences coupling efficiency. Aprotic solvents such as dichloromethane, tetrahydrofuran, or dimethylformamide typically provide optimal results by maintaining reactant solubility while minimizing competing reactions. Temperature control also proves crucial, with many protocols initiating the reaction at lower temperatures (0-5°C) before allowing gradual warming to room temperature as the activated species forms and subsequently reacts with the amine.
The reaction progress can be monitored through thin-layer chromatography (TLC) or more advanced analytical techniques such as HPLC or LC-MS. These methods allow chemists to track the disappearance of starting materials and the emergence of the desired amide product. Complete conversion typically requires several hours, though reaction times vary based on specific reagents, concentrations, and conditions employed.
Purification of the final capsaicin or nonivamide product generally involves a combination of techniques. Initial workup procedures remove water-soluble byproducts and excess reagents through aqueous extractions. Subsequent chromatographic purification, either through traditional column chromatography or more advanced techniques like preparative HPLC, isolates the target molecule from any remaining impurities. For pharmaceutical or analytical grade nonivamide powder, recrystallization serves as a final purification step to achieve the highest purity standards.
Rebecca: Synthetic Capsaicin Manufacturer
The synthesis of capsaicin and its analogs demonstrates the remarkable capabilities of modern organic chemistry to reproduce and even enhance nature's molecules. Through carefully orchestrated sequences of reactions, chemists can construct these complex structures from simpler building blocks, controlling reactivity and stereochemistry at each step. Whether pursuing the natural compound or synthetic variants like nonivamide powder, these synthetic pathways provide access to important molecules with applications spanning multiple industries and research fields.
The laboratory synthesis offers significant advantages over natural extraction methods, including consistent quality, scalable production, and the ability to create structural modifications that may improve stability or biological activity for specific applications. As analytical techniques and synthetic methodologies continue to advance, we can anticipate even more efficient and environmentally friendly approaches to capsaicinoid synthesis in the future, further expanding the utility of these fascinating compounds.
Rebecca is a synthetic capsaicin supplier offering premium-quality products for research, pharmaceutical, and industrial applications. Our capsaicin meets the highest standards with specifications of ≥98% purity confirmed by HPLC analysis. With an impressive Scoville Heat Unit rating of 16,000,000 SHU, our product delivers exceptional potency and consistency for your capsaicinoid needs.
We understand the importance of quality assurance in your projects, which is why we provide comprehensive documentation, including Certificates of Analysis (COA) and Material Safety Data Sheets (MSDS) with every order. Our dedication to quality control ensures that each batch of nonivamide powder meets stringent specifications for purity and performance.
Free samples are available upon request for qualified clients to evaluate our products before making larger commitments. Our technical experts are ready to assist with any questions regarding applications, handling procedures, or custom specifications to meet your particular requirements.
For more information or to place an order, please reach out to us at information@sxrebecca.com.
References
- Kaga, H., Miura, M., & Orito, K. "A Facile Procedure for Synthesis of Capsaicin." Journal of Organic Chemistry, 1989, 54(13), 3477-3478.
- Appendino, G., Minassi, A., & Collado, J.A. "Capsaicinoids and Capsinoids. An Overview of Natural Capsaicin Analogues." Current Drug Targets, 2011, 12(1), 115-121.
- Castillo, E., Torres-Gavilán, A., Severiano, P., Arturo, N., & López-Munguía, A. "Enzymatic Synthesis of Capsaicin Analogs and Their Effect on the T-Type Ca2+ Channels." Biochemical and Biophysical Research Communications, 2007, 356(2), 424-430.
- Kobata, K., Kawamura, M., Toyoshima, M., Tamura, Y., Ogawa, S., & Watanabe, T. "Lipase-catalyzed Synthesis of Capsaicin Analogs by Amidation of Vanillylamine with Fatty Acid Derivatives." Biotechnology Letters, 1998, 20(5), 451-454.
- Bradshaw, H.B., Fioravanti, B., Patwardhan, A., Mangione, R., & Burstein, S.H. "Synthesis of Capsaicin Analogues, and Their Biological Evaluation Against Pain Targets." Bioorganic & Medicinal Chemistry Letters, 2005, 15(15), 3585-3589.








