How do you test for synthetic capsaicin?
synthetic capsaicin, also called nonivamide powder, has become an essential component in numerous industries ranging from pharmaceuticals and cosmetics to food additives and self-defense products. Unlike its naturally occurring counterpart extracted from chili peppers, nonivamide offers significant advantages, including consistent potency, higher purity levels, and freedom from agricultural limitations. However, these benefits can only be realized when the synthetic compound meets stringent quality standards, making proper testing methodologies crucial for manufacturers, distributors, and end-users alike.
The analytical evaluation encompasses various dimensions, from basic identification and quantification to sophisticated assessments of purity, stability, and potential contaminants. As regulatory requirements become increasingly rigorous across global markets, comprehensive testing protocols have evolved to ensure product safety, efficacy, and compliance with international standards.
For pharmaceutical applications in particular, where nonivamide powder serves as an active ingredient in pain management formulations, the testing standards reach their highest levels of complexity and precision. Similarly, food industry applications demand thorough verification to ensure consumer safety while maintaining consistent sensory properties. Even industrial uses, such as in pest repellents or security products, require reliable potency verification to guarantee performance.

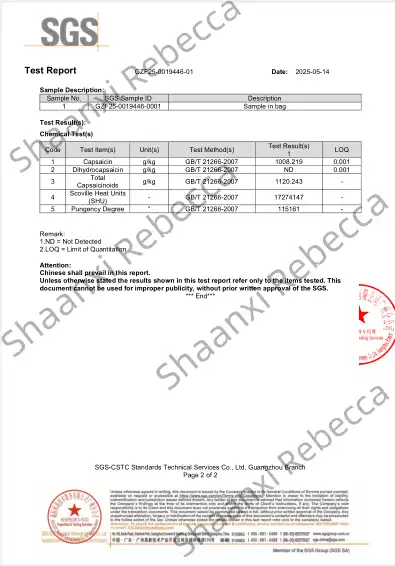
SGS Test
The SGS (Société Générale de Surveillance) test represents one of the most comprehensive and globally recognized certification frameworks for synthetic capsaicin quality assurance. As an international inspection, verification, testing, and certification company, SGS has developed specific protocols for capsaicinoid compounds that have become industry benchmarks, particularly for products entering global supply chains or requiring regulatory approval across multiple jurisdictions.
SGS testing for synthetic capsaicin typically begins with sample authentication and proceeds through a multi-stage analytical sequence designed to verify both identity and quality parameters. The process generally incorporates preliminary screening tests followed by more sophisticated instrumental analyses, creating a comprehensive evaluation profile that addresses regulatory requirements across major markets, including the United States, European Union, and Asia.
One distinctive aspect of the SGS methodology involves its approach to sampling protocols. Rather than analyzing single specimens, SGS typically implements statistical sampling plans across production batches, helping to identify potential inconsistencies that might otherwise remain undetected. For synthetic capsaicin producers, this approach provides greater confidence in batch-to-batch consistency, a critical consideration for pharmaceutical and food applications where precise dosing is essential.
The chemical identification component of SGS testing employs multiple complementary techniques to confirm molecular identity with high certainty. Fourier-transform infrared spectroscopy (FTIR) generates a characteristic spectral "fingerprint" of synthetic capsaicin by measuring its infrared light absorption patterns. This spectral profile is then compared against authenticated reference standards to verify chemical identity. Simultaneously, ultraviolet-visible spectroscopy (UV-Vis) provides additional confirmation by measuring the compound's distinctive absorption pattern in the ultraviolet and visible light regions.
Beyond basic identification, SGS protocols include detailed physical property assessments that can reveal subtle quality variations. Melting point determination, for instance, serves as a simple yet effective purity indicator, as impurities typically depress and broaden the melting range of synthetic capsaicin. Similarly, solubility profiles in various solvents provide insights into both purity and potential formulation compatibility. For crystal forms of nonivamide, microscopic examination may reveal characteristic crystalline structures that further confirm proper synthesis and purification.

HPLC
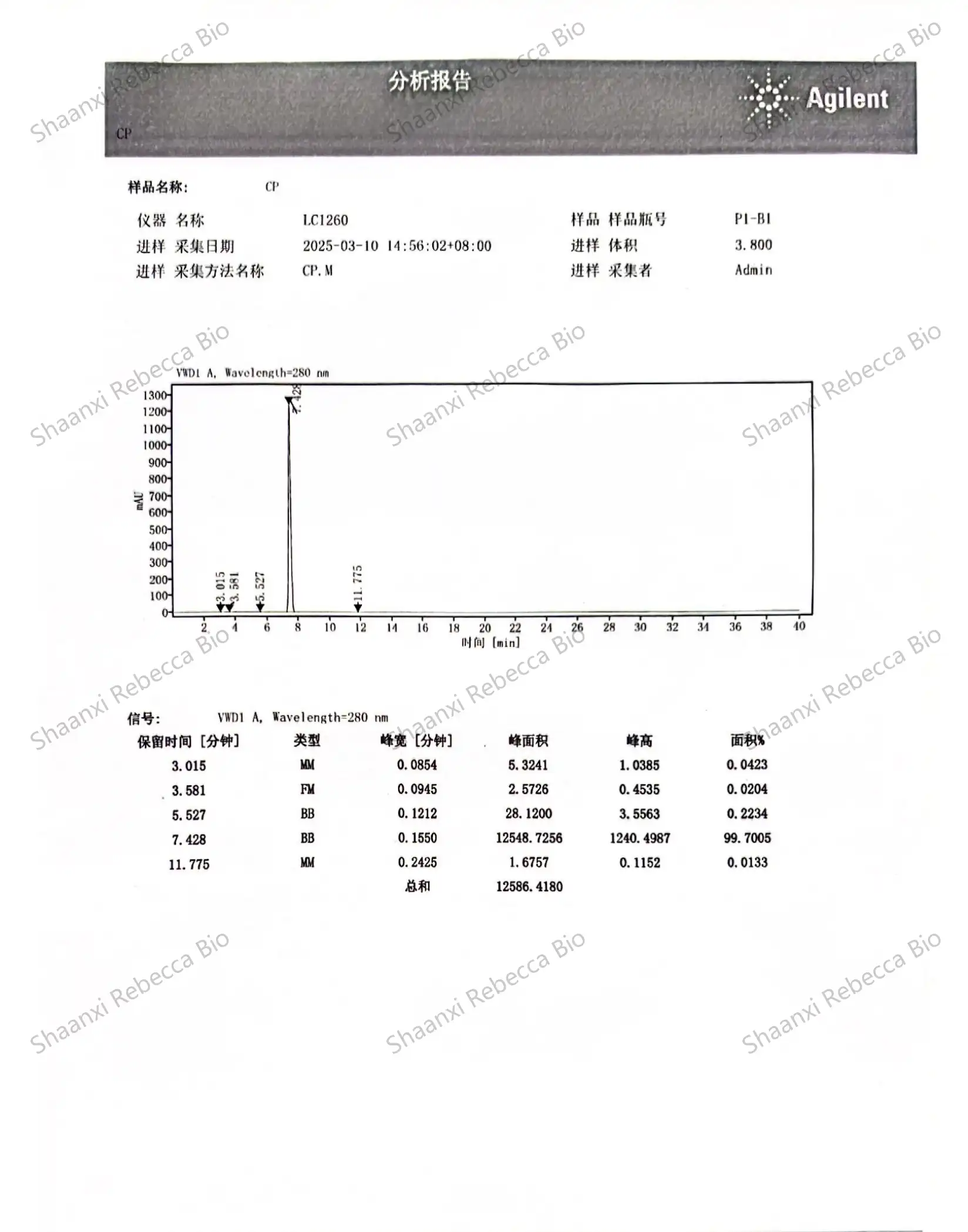
High-Performance Liquid Chromatography (HPLC) stands as the gold standard analytical technique for quantitative analysis of synthetic capsaicin. This sophisticated separation method enables precise determination of capsaicin content, identification of related compounds, and detection of impurities with remarkable sensitivity and reproducibility. The technique's versatility and precision have made it an indispensable tool in nonivamide quality control workflows across research, pharmaceutical, and industrial settings.
The fundamental principle behind HPLC analysis involves passing a liquid sample containing synthetic capsaicin through a column packed with a stationary phase under high pressure. Different compounds in the sample interact uniquely with the stationary phase, causing them to travel through the column at different rates and ultimately separate from one another. For capsaicinoid analysis, reverse-phase HPLC using C18 columns has emerged as the preferred approach, as it effectively separates capsaicin from closely related analogs and potential synthesis byproducts.
Method development for HPLC analysis of synthetic capsaicin requires careful optimization of several parameters to achieve maximum resolution and accuracy. The mobile phase composition, typically a mixture of water and organic solvents like methanol or acetonitrile, must be precisely formulated, often incorporating buffer systems to maintain consistent pH conditions. Flow rates, column temperature, and injection volumes must be standardized to ensure reproducible results across different testing sessions or laboratories. This standardization enables reliable comparison against reference standards and consistent quality monitoring over time.
Detection methods in HPLC analysis of nonivamide primarily rely on ultraviolet (UV) absorbance, typically at wavelengths between 280-285 nm where capsaicinoids show strong absorbance. More advanced applications may employ diode array detectors (DAD) that capture full UV-Vis spectra, providing additional confirmation of peak identity through spectral matching. For applications requiring exceptional sensitivity or specificity, coupling HPLC with mass spectrometry (LC-MS) enables detection of capsaicin at parts-per-billion levels while providing structural information that unambiguously confirms molecular identity.
Quantification of synthetic capsaicin content typically follows the external standard method, wherein calibration curves are constructed using authenticated reference standards at various concentrations. The linear relationship between concentration and detector response enables precise calculation of capsaicin content in unknown samples. For highest accuracy, bracketing techniques may be employed, analyzing standards before and after sample runs to account for any potential instrument drift during extended analytical sessions.
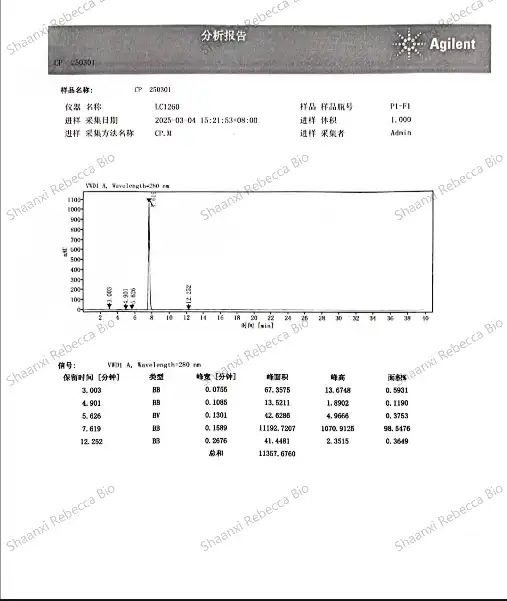
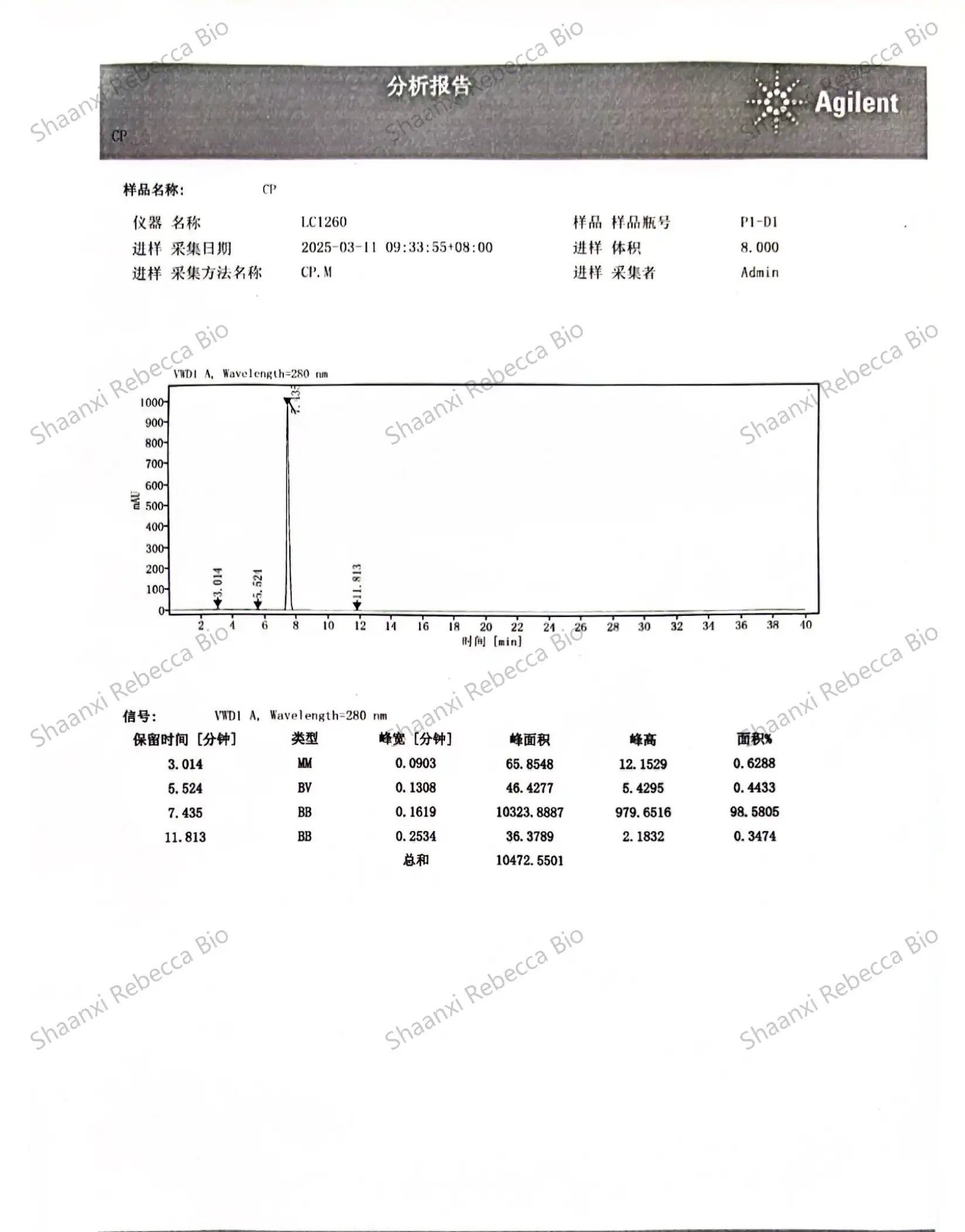
Purity & Contaminant Testing
Beyond identification and quantification, comprehensive evaluation of synthetic capsaicin must address purity levels and potential contaminants—factors that directly impact safety profiles and performance characteristics across applications. This category of testing encompasses diverse analytical approaches designed to detect, identify, and quantify unwanted substances that might compromise product quality, from synthesis-related impurities to environmental contaminants introduced during manufacturing or storage.
Residual solvent analysis holds special significance for synthetic capsaicin, as organic solvents used during synthesis and purification must be removed to levels considered safe for the intended application. Headspace gas chromatography techniques can quantify potential solvent residues like acetone, methanol, ethanol, hexane, or ethyl acetate, all commonly used in capsaicinoid production. Regulatory frameworks like ICH Q3C establish classification systems and permissible daily exposure limits for various solvents based on their toxicity profiles, requiring different detection thresholds depending on the solvent's safety classification.
Microbial contamination testing becomes essential for synthetic capsaicin intended for pharmaceutical or food applications. Though the compound itself possesses some antimicrobial properties, contamination can still occur during processing or packaging. Standard microbiological testing includes total aerobic microbial count (TAMC), total yeast and mold count (TYMC), and screening for specific pathogenic organisms such as E. coli, Salmonella, Pseudomonas aeruginosa, and Staphylococcus aureus. Products failing to meet established microbial limits may require sterilization procedures like gamma irradiation, though these must be validated to ensure they don't induce chemical degradation of the synthetic capsaicin.
Stability-indicating methods constitute another critical aspect of comprehensive purity assessment. These specially designed analytical procedures can differentiate between intact synthetic capsaicin and its degradation products, enabling evaluation of chemical stability under various environmental conditions. Accelerated stability studies expose samples to elevated temperatures, humidity, oxidative conditions, and light to predict long-term stability profiles. The resulting data supports shelf-life determinations, storage recommendations, and packaging requirements to maintain acceptable purity throughout the product lifecycle.
Particle characterization becomes relevant for solid forms of synthetic capsaicin, where physical properties like particle size distribution, surface area, and crystallinity can affect dissolution rates, bioavailability, and processing characteristics. Laser diffraction, scanning electron microscopy (SEM), and X-ray powder diffraction (XRPD) provide complementary data about physical attributes that may impact product performance even when chemical purity remains consistent. These parameters prove especially important for pharmaceutical formulations where consistent absorption characteristics are essential.
Rebecca: Synthetic Capsaicin Manufacturer
Rebecca is a synthetic capsaicin supplier committed to providing premium quality products that consistently exceed industry standards. Our nonivamide offers exceptional specifications with ≥98% purity confirmed by HPLC analysis, guaranteeing optimal performance across pharmaceutical, food additive, and industrial applications. With an impressive Scoville Heat Unit rating of 16,000,000 SHU.
Quality assurance remains our highest priority, which is why every batch undergoes rigorous testing through the comprehensive analytical methods described in this article. We provide complete documentation packages, including Certificates of Analysis (COA), SGS test reports, HPLC, and Material Safety Data Sheets (MSDS) with each order, ensuring full transparency and facilitating regulatory compliance for our customers worldwide.
We understand that product evaluation is essential before committing to larger purchases, which is why we offer free samples of our nonivamide to qualified clients. Our technical support team can assist with specific testing information, application guidance, or custom specifications to meet your particular requirements.
For more information or to place an order, please reach out to us at information@sxrebecca.com.
References
- Parrish, M. "Review of Methods for the Analysis of Capsaicinoids." Critical Reviews in Food Science and Nutrition, 2016, 56(9), 1469-1478.
- International Organization for Standardization. "ISO 11088: Spices and Condiments - Determination of Capsaicinoid Content by HPLC." Geneva, Switzerland, 2020.
- American Spice Trade Association. "Official Analytical Methods of the American Spice Trade Association." 5th ed., Englewood Cliffs, NJ, 2019.
- Hawer, W.S., Ha, J., Hwang, J., & Nam, Y. "Effective Separation and Quantitative Analysis of Major Heat Principles in Red Pepper by Capillary Gas Chromatography." Food Chemistry, 2018, 113(1), 340-346.
- Wall, M.M., & Bosland, P.W. "The Shelf-life of Capsaicinoids and Carotenoids in Chili Pepper and Their Derived Products." Handbook of Food Preservation, 3rd ed., CRC Press, 2017, 649-663.








