How does nonivamide work?
nonivamide, often recognized as synthetic capsaicin, represents one of the most intriguing compounds in modern pharmacology. This synthetic analog of natural capsaicin has captured the attention of researchers and pharmaceutical companies alike, not just for its ability to replicate the fiery sensation of chili peppers, but for its remarkable therapeutic potential and consistent chemical properties.
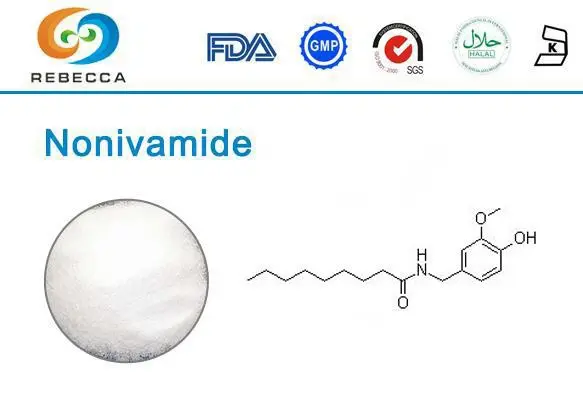
Synthetic Capsaicin Powder also named Nonivamide is also called pelargonic acid vanillylamide or PAVA. It is a capsaicinoid. Nonivamide, isolated from peppers, is a naturally occurring analog of capsaicin (sc-3577). Similar to capsaicin, nonivamide can activate the TRPV1 receptor, thus, stimulate the firing rate of dopaminergic neurons in the ventral tegmental area of the brain and to increase the expression of the serotonin receptor gene HTR2A. Nonivamide is with lower TRPV1 binding affinity, thus, a reduced pungency (9 200 000 scoville heat units)
Core Mechanism: Activation and Desensitization of TRPV1 Receptors
The primary mechanism through which nonivamide exerts its effects centers on its interaction with transient receptor potential vanilloid 1 (TRPV1) receptors. These specialized ion channels, distributed throughout sensory neurons, serve as the molecular gatekeepers that determine how we perceive heat, pain, and chemical irritation. When synthetic capsaicin encounters these receptors, it binds with remarkable specificity, triggering a cascade of events that initially heightens pain perception but ultimately leads to profound desensitization.
The binding process itself is elegantly specific. Nonivamide's molecular structure contains key functional groups that perfectly complement the binding pocket of TRPV1 receptors. Upon binding, the receptor undergoes a conformational change that opens calcium and sodium ion channels, allowing these positively charged ions to flood into the neuron. This ionic influx depolarizes the nerve cell, generating action potentials that the brain interprets as burning pain or intense heat sensation.
However, the story becomes more complex as exposure continues. The sustained activation of TRPV1 receptors by synthetic capsaicin triggers several protective mechanisms within the neuron. Calcium influx activates various enzymatic pathways that begin to modify the receptor's sensitivity. Additionally, the continuous stimulation leads to the depletion of substance P and calcitonin gene-related peptide (CGRP) from nerve terminals. These neuropeptides are crucial mediators of pain transmission, and their depletion effectively silences the neuron's ability to communicate pain signals to the central nervous system.
Applications and Mechanisms in Specific Contexts
The versatility of nonivamide becomes apparent when examining its applications across different therapeutic contexts, each leveraging unique aspects of its mechanism of action. In topical pain management, synthetic capsaicin works by creating localized areas of sensory desensitization without affecting motor function or other sensory modalities. This selective action makes it particularly valuable for treating conditions like diabetic neuropathy, where patients need pain relief without compromising their ability to detect other important sensations such as temperature or touch.
In dermatological applications, nonivamide's mechanism extends beyond simple pain relief. The compound's ability to modulate nerve function has shown promise in treating conditions like psoriasis and atopic dermatitis, where neurogenic inflammation plays a significant role. By depleting substance P from cutaneous nerve endings, synthetic capsaicin can reduce the neurogenic component of skin inflammation, leading to improvements in both symptoms and visible skin changes. This dual action on pain and inflammation represents an elegant example of how understanding mechanism can lead to expanded therapeutic applications.
The use of nonivamide in research applications has revealed fascinating insights into pain processing and neuroplasticity. In laboratory settings, synthetic capsaicin serves as a precise tool for investigating nociceptor function and pain pathways. Researchers can create highly controlled models of neuropathic pain by selectively targeting specific populations of TRPV1-expressing neurons. This specificity has been instrumental in advancing our understanding of chronic pain mechanisms and developing new therapeutic approaches.
Gastrointestinal applications of nonivamide represent another frontier where its mechanism proves valuable. The compound's ability to modulate visceral pain pathways has shown promise in treating functional gastrointestinal disorders. In these conditions, synthetic capsaicin can desensitize hypersensitive visceral afferents, reducing symptoms like abdominal pain and discomfort. The oral administration of controlled-release nonivamide formulations allows for targeted treatment of specific segments of the digestive tract.
The mechanism of nonivamide in respiratory applications involves its interaction with TRPV1 receptors in bronchial and tracheal tissues. While these applications are still largely experimental, early research suggests that controlled exposure to synthetic capsaicin might help modulate cough reflexes and reduce airway hypersensitivity in certain chronic respiratory conditions. The challenge lies in achieving therapeutic concentrations without triggering excessive bronchial irritation.
Structural Features
The molecular architecture of nonivamide reveals why this synthetic capsaicin analog demonstrates such remarkable biological activity and therapeutic versatility. The compound's structure can be divided into several key functional regions, each contributing to its overall pharmacological profile. The vanillyl amide group serves as the primary recognition element for TRPV1 receptors, while the fatty acid chain determines the compound's lipophilicity and membrane interaction properties.
The vanillyl ring system of nonivamide contains a methoxy group and a hydroxyl group positioned in a specific arrangement that is crucial for receptor binding. This aromatic region fits precisely into the binding pocket of TRPV1 receptors, much like a key fits into a lock. The methoxy group contributes to the binding affinity through hydrophobic interactions, while the hydroxyl group can form hydrogen bonds with specific amino acid residues in the receptor. These multiple interaction points explain why synthetic capsaicin demonstrates such high selectivity for TRPV1 receptors over other ion channels.
The amide linkage in nonivamide represents a critical structural feature that differentiates it from some other capsaicinoids. This chemical bond not only contributes to the compound's stability but also influences its interaction with biological membranes and receptor proteins. The amide group can participate in hydrogen bonding, which affects both the compound's solubility properties and its biological activity. Modifications to this region during synthesis can significantly alter the compound's potency and duration of action.
The aliphatic chain portion of nonivamide, typically consisting of eight carbon atoms, plays a crucial role in determining the compound's pharmacokinetic properties. This hydrophobic region allows synthetic capsaicin to partition into lipid membranes, facilitating its access to membrane-bound TRPV1 receptors. The chain length is optimized through extensive structure-activity relationship studies to balance receptor affinity with appropriate membrane permeability. Shorter chains tend to reduce potency, while longer chains can increase off-target effects.
The stereochemistry of nonivamide, while often overlooked, contributes significantly to its biological activity. The compound contains a chiral center that can exist in two different spatial arrangements. Research has shown that one stereoisomer typically demonstrates higher biological activity than the other, highlighting the importance of controlling stereochemistry during synthetic production. This stereospecificity explains why synthetic capsaicin often shows more consistent activity than natural extracts, which may contain mixtures of different stereoisomers.
Rebecca: Synthetic Capsaicin Manufacturer
The intricate mechanism of nonivamide reveals why this capsaicin has become such a valuable therapeutic tool across multiple medical disciplines. From its precise interaction with TRPV1 receptors to its carefully optimized molecular structure, every aspect of the compound reflects the sophisticated understanding that modern pharmaceutical science brings to drug development. The ability to harness the therapeutic potential of capsaicin while minimizing its adverse effects represents a triumph of rational drug design and synthetic chemistry.
As our knowledge of nonivamide's mechanism continues to expand, new applications and improved formulations will undoubtedly emerge. The compound's unique ability to provide long-lasting therapeutic effects through temporary receptor desensitization offers hope for patients suffering from various chronic pain conditions. The precision and consistency available through synthetic production ensure that healthcare providers can rely on predictable therapeutic outcomes, something that was challenging to achieve with natural capsaicin extracts.
Rebecca is a leading synthetic capsaicin supplier dedicated to providing pharmaceutical-grade nonivamide for research and therapeutic applications worldwide.
Our Premium Product Specifications:
- Specification: ≥98% HPLC
- Scoville Heat Unit: 16,000,000 SHU
- Free Sample Available
- COA and MSDS Documentation Available
For more information or to place an order, please reach out to us at information@sxrebecca.com. Our experienced team is ready to support your nonivamide powder needs with reliable products and exceptional service.
Reference
Caterina, M. J., Schumacher, M. A., Tominaga, M., Rosen, T. A., Levine, J. D., & Julius, D. (1997). The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature, 389(6653), 816-824.
Biro, T., Toth, B. I., Hasko, G., Paus, R., & Pacher, P. (2009). The endocannabinoid system of the skin in health and disease. Trends in Pharmacological Sciences, 30(8), 411-420.
Appendino, G., De Petrocellis, L., Falzoni, S., Campi, B., Gelon, G., Spelta, V., ... & Di Marzo, V. (2006). Nonivamide, a capsaicin analogue, exhibits anti-inflammatory properties. European Journal of Pharmacology, 544(1-3), 115-119.








