Synthetic Capsaicin VS Acetic Acid
The world of biochemical compounds is vast, with numerous substances playing crucial roles in various industries ranging from pharmaceuticals to food science. Among these compounds, synthetic capsaicin (nonivamide powder)and acetic acid stand out as two distinct chemical entities with unique properties and applications. While they may seem unrelated at first glance, understanding their differences and similarities provides valuable insights into their industrial and scientific significance. This comprehensive comparison explores the fundamental aspects of these compounds, shedding light on their structure, properties, applications, and mechanisms of action.
nonivamide powder, a laboratory-produced version of the natural compound found in chili peppers, has gained substantial attention across multiple industries due to its remarkable properties. Unlike naturally occurring capsaicin, the synthetic variant offers consistent potency and purity, making it highly valuable for precise applications. Meanwhile, acetic acid, commonly recognized as the main component of vinegar, has been a staple in human civilization for centuries, serving purposes ranging from food preservation to industrial manufacturing.

Chemical Structure & Properties
Understanding the fundamental chemical structure and properties of synthetic capsaicin and acetic acid is essential for appreciating their distinct behaviors and applications. These compounds differ significantly in their molecular composition, physical characteristics, and chemical reactivity, which directly influences their utility across various industries.
Synthetic Capsaicin Structure
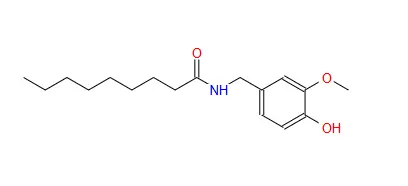
Synthetic capsaicin (N-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methylnon-6-enamide) is a precisely engineered compound designed to replicate the chemical structure of natural capsaicin found in hot peppers. With a molecular formula of C18H27NO3, this compound features a vanillyl group connected to a fatty acid chain through an amide bond. This specific arrangement is responsible for its characteristic properties, particularly its ability to interact with pain receptors.
The synthetic version offers several advantages over natural capsaicin, including exceptional purity (≥98% by HPLC analysis), consistent potency, and freedom from agricultural contaminants. While natural capsaicin extract may contain varying levels of other capsaicinoids and plant compounds, synthetic capsaicin provides precise concentration, making it ideal for applications requiring exact dosing and reliable results.
A remarkable feature of nonivamide powder is its extreme pungency, measured at approximately 16,000,000 Scoville Heat Units (SHU). For perspective, this is significantly higher than even the hottest natural peppers, with the Carolina Reaper averaging around 1.5 million SHU. This extraordinary potency means that even minute quantities of synthetic capsaicin can produce substantial effects, making careful handling essential.
Physically, synthetic capsaicin appears as a white to off-white crystalline powder with minimal solubility in water but good solubility in organic solvents such as ethanol, methanol, and acetone. It exhibits a melting point of approximately 65°C and remains stable under normal storage conditions when protected from light and moisture.
Acetic Acid Structure
Acetic acid (CH3COOH), in contrast, possesses a much simpler molecular structure consisting of a methyl group attached to a carboxylic acid functional group. This straightforward composition gives acetic acid its characteristic properties as a weak organic acid. In its pure form, acetic acid exists as a colorless liquid with a sharp, pungent odor familiar to anyone who has encountered vinegar, which typically contains 4-8% acetic acid in water.
With a molecular weight of just 60.05 g/mol (compared to synthetic capsaicin's approximately 305.4 g/mol), acetic acid is a substantially smaller molecule. It features a melting point of 16.6°C and a boiling point of 118.1°C under standard conditions. Unlike synthetic capsaicin, acetic acid is highly water-soluble, forming hydrogen bonds with water molecules that facilitate its dissolution.
Acetic acid exhibits moderate acidity with a pKa value of around 4.76, making it stronger than most carboxylic acids but considerably weaker than mineral acids like hydrochloric or sulfuric acid. This moderate acidity allows it to participate in various chemical reactions, including esterification, neutralization, and condensation processes, contributing to its versatility in industrial applications.
Primary Uses
The vastly different chemical structures and properties of synthetic capsaicin and acetic acid naturally lead to diverse applications across multiple industries. Each compound has carved out specific niches where its unique characteristics provide particular advantages.
Synthetic Capsaicin Applications
Synthetic capsaicin has found numerous valuable applications, primarily in the pharmaceutical, agricultural, and personal security sectors. Its precise formulation and consistent potency make it particularly valuable when exact dosing is essential.
In the pharmaceutical industry, synthetic capsaicin serves as a key ingredient in topical analgesics for pain management. These products provide relief for conditions such as arthritis, diabetic neuropathy, and post-herpetic neuralgia by initially stimulating and then desensitizing pain receptors. High-concentration patches containing 8% nonivamide powder have received FDA approval for treating neuropathic pain, demonstrating the compound's therapeutic value. The ability to precisely control the concentration ensures consistent efficacy and safety profiles in these medical applications.
Beyond pain management, research into nonivamide continues to explore its potential in weight management products and thermogenic supplements. Some studies suggest that capsaicin can temporarily increase metabolic rate and reduce appetite, though more research is needed to fully establish these effects. The controlled potency of synthetic capsaicin allows for precise formulation in these investigational applications.
In agricultural applications, synthetic capsaicin serves as an effective and non-toxic repellent against mammals and certain insects. Products containing this compound help farmers protect crops from wildlife damage without resorting to more harmful chemical pesticides. The extreme potency means that even highly diluted formulations can effectively deter pests while minimizing environmental impact.
Perhaps most visibly, synthetic capsaicin constitutes the active ingredient in many personal defense sprays and animal repellents. Its powerful effect on mucous membranes and pain receptors creates a temporary but intense incapacitating effect when deployed in self-defense situations. The controlled synthesis process ensures that these products deliver consistent performance in critical situations where reliability is paramount.

Acetic Acid Applications
Acetic acid, with its different chemical profile, serves an entirely different set of industries and purposes. Its widespread availability and well-understood properties have made it a staple in numerous processes for centuries.
In the food industry, acetic acid (primarily in the form of vinegar) functions as a preservative, flavoring agent, and acidulant. Its antimicrobial properties help extend the shelf life of many food products by inhibiting the growth of bacteria, yeasts, and molds. The distinct flavor profile of acetic acid also contributes to numerous culinary traditions worldwide, from salad dressings to pickled vegetables.
The chemical industry heavily relies on acetic acid as a reagent and solvent in the production of various compounds. It serves as a precursor in manufacturing vinyl acetate monomer (essential for adhesives and paints), acetic anhydride (used in aspirin production), and various acetate esters employed in solvents and fragrances. The relatively simple structure and predictable reactivity of acetic acid make it an ideal building block for these processes.
In household applications, diluted acetic acid solutions shine as effective cleaning agents. The mild acidity helps dissolve mineral deposits, remove grease, and kill certain household bacteria. Its relative safety compared to harsher cleaning chemicals has contributed to its popularity in eco-friendly cleaning products.
The textile industry employs acetic acid in dyeing processes, where it helps fix colors to fabrics. Similarly, the printing industry uses it in certain ink formulations. In both cases, the ability of acetic acid to create mildly acidic conditions without causing significant damage to materials proves valuable.

Mechanism of Action
Perhaps the most fascinating aspect of comparing synthetic capsaicin and acetic acid lies in understanding their drastically different mechanisms of action. These mechanisms explain why these compounds produce such distinct effects despite both being considered irritants in certain contexts.
Synthetic Capsaicin's Neurological Pathway
Synthetic capsaicin operates through a highly specific biochemical pathway involving direct interaction with sensory neurons. The compound's primary mechanism revolves around its ability to selectively bind to and activate the transient receptor potential vanilloid 1 (TRPV1) receptors, also known as capsaicin receptors. These specialized ion channels are predominantly found in nociceptive neurons, cells specifically responsible for detecting potentially harmful stimuli.
When nonivamide powder binds to TRPV1 receptors, it triggers the opening of these calcium channels, allowing an influx of calcium ions into the neuron. This sudden ion influx creates an action potential, sending signals to the brain that are interpreted as an intense burning sensation. This neurological pathway explains why even minute quantities of nonivamide can produce such powerful sensory effects—the compound essentially "tricks" the nervous system into perceiving tissue damage and extreme heat where none actually exists.
What makes synthetic capsaicin particularly interesting from a therapeutic perspective is its biphasic effect. Initial exposure causes activation of the TRPV1 receptors and the associated pain sensation, but prolonged or repeated exposure leads to a phenomenon known as desensitization. During this phase, the affected neurons become temporarily unresponsive not only to capsaicin but also to various other painful stimuli. This desensitization forms the basis for capsaicin's paradoxical use as an analgesic in certain pain management applications.
The neurological specificity of synthetic capsaicin extends beyond pain perception. TRPV1 activation also triggers the release of substance P and other neuropeptides from sensory neurons, leading to localized inflammatory responses including vasodilation, increased vascular permeability, and activation of inflammatory cells. This cascade explains the redness, swelling, and warmth that often accompany significant capsaicin exposure.
Research has shown that synthetic capsaicin, due to its precise chemical structure, binds to TRPV1 receptors with exceptional affinity, making it substantially more potent than many of its natural counterparts. This heightened receptor binding efficiency contributes to its extreme Scoville Heat Unit rating and makes it particularly valuable in applications requiring reliable, consistent receptor activation.
Acetic Acid's Chemical Interactions
Acetic acid, by contrast, operates through fundamentally different biochemical mechanisms. As a weak acid, its primary interactions stem from its ability to donate protons (H+ ions) in aqueous solutions, creating acidic conditions. This proton donation forms the basis for most of acetic acid's biological and chemical effects.
When acetic acid comes into contact with biological tissues, it partially dissociates into acetate ions (CH3COO-) and hydrogen ions (H+). These hydrogen ions can temporarily disrupt local pH balance, affecting various cellular processes. Unlike synthetic capsaicin, which targets specific receptors, acetic acid produces more generalized effects through this acid-base chemistry.
In concentrated forms, acetic acid can denature proteins through acidification, disrupting their three-dimensional structure and consequently their function. This property contributes to its antimicrobial effects—by denaturing essential proteins in bacterial cell membranes and enzymes, acetic acid can inhibit microbial growth or even cause cell death. However, this mechanism requires relatively high concentrations compared to the trace amounts of synthetic capsaicin needed to produce biological effects.
When inhaled or tasted, acetic acid stimulates chemoreceptors that respond to acidic conditions rather than specific pain receptors. These receptors signal the presence of sour substances, creating the characteristic sensory experience associated with vinegar. Additionally, the volatility of acetic acid allows it to stimulate olfactory receptors, contributing to its distinctive aroma.
In industrial applications, acetic acid's mechanism centers around its carboxylic acid functionality, which enables reactions such as esterification, where it combines with alcohols to form acetate esters. This reactivity forms the basis for its extensive use as a chemical intermediate in various manufacturing processes.
Rebecca: Synthetic Capsaicin Manufacturer
When seeking a reliable source for this specialized compound, Rebecca Bio-Tech stands as a premier synthetic capsaicin supplier committed to providing products of exceptional quality and purity.
Our products meet the highest industry standards with a purity specification of ≥98% confirmed by HPLC analysis. With an impressive Scoville Heat Unit rating of 16,000,000 SHU, our product delivers consistent potency for your pharmaceutical, agricultural, or industrial applications.
At Rebecca Bio-Tech, we understand the importance of comprehensive documentation and support for your regulatory and safety requirements. That's why we provide detailed Certificate of Analysis (COA) and Material Safety Data Sheets (MSDS) with every order, ensuring you have all the information needed for proper handling and compliance.
We believe in helping our customers make informed decisions, which is why we offer free samples of our nonivamide powder. This allows you to verify our product's quality and suitability for your specific applications before making a larger commitment.
For more information about our nonivamide or to place an order, please reach out to our knowledgeable team at information@sxrebecca.com. Our specialists are ready to answer your questions and provide personalized support for your nonivamide needs.
References
1. Basith, S., Cui, M., Hong, S., & Choi, S. (2016). Harnessing the therapeutic potential of capsaicin and its analogues in pain and other diseases. Molecules, 21(8), 966.
2. Fattori, V., Hohmann, M. S., Rossaneis, A. C., Pinho-Ribeiro, F. A., & Verri, W. A. (2016). Capsaicin: Current understanding of its mechanisms and therapy of pain and other pre-clinical and clinical uses. Molecules, 21(7), 844.
3. O'Neill, J., Brock, C., Olesen, A. E., Andresen, T., Nilsson, M., & Dickenson, A. H. (2012). Unravelling the mystery of capsaicin: a tool to understand and treat pain. Pharmacological Reviews, 64(4), 939-971.
4. Ciriminna, R., Meneguzzo, F., Delisi, R., & Pagliaro, M. (2017). Citric acid: emerging applications of key biotechnology industrial product. Chemistry Central Journal, 11(1), 22.
5. Budak, N. H., Aykin, E., Seydim, A. C., Greene, A. K., & Guzel‐Seydim, Z. B. (2014). Functional properties of vinegar. Journal of Food Science, 79(5), R757-R764.








