DL-6 8-Thioctic Acid VS R Alpha-Lipoic-Acid
In the realm of antioxidants and metabolic support supplements, alpha-lipoic acid (ALA) has emerged as a standout compound, celebrated for its unique dual solubility and versatile biological effects. Yet, beneath the umbrella term "alpha-lipoic acid" lies a critical distinction: the natural R-enantiomer (R alpha-lipoic acid) and the synthetic racemic mixture (dl-6 8-thioctic acid). This distinction is not merely chemical; it directly impacts bioactivity, therapeutic potential, and safety. For consumers and healthcare practitioners alike, understanding these differences is essential to making informed decisions about supplementation.

DL-6 8-Thioctic Acid
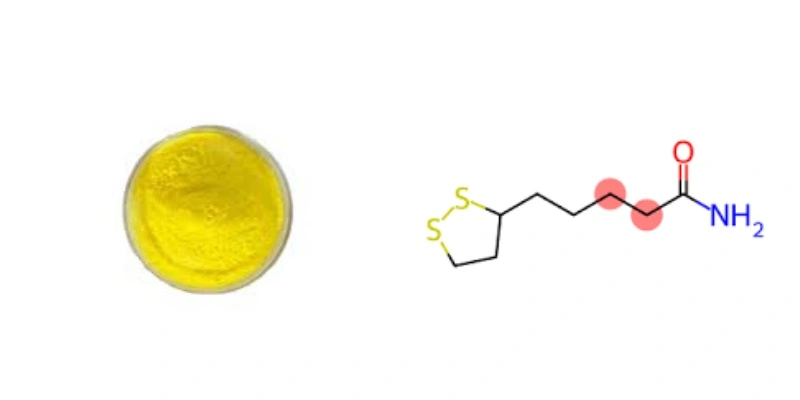
【English name】: Lipoic acid powder
【Latin Name】: 1,2-Dithiolane-3-pentanoic acid;
【CAS No.】: 1077-28-7
【Molecular Formula】:C8H14O2S2
【Specification】: Lipoic acid99%
【Appearance】: Light yellow powder
【Mesh size】:80 Mesh
【Test Method】: HPLC
Difference: Enantiomers and Their Origins
To grasp the divergence between DL-6 8-thioctic acid and R alpha-lipoic acid, we must first examine their molecular structure. Both compounds share the same chemical formula (C₈H₁₄O₂S₂) but differ in the spatial arrangement of atoms around a central chiral carbon, a characteristic that creates enantiomers, molecules that are mirror images of one another.
R Alpha-Lipoic Acid: The "natural" enantiomer, denoted as (R)-(+)-α-lipoic acid, is the only form biosynthesized in human cells and most other organisms. It functions as an essential cofactor for four mitochondrial enzyme complexes critical to aerobic metabolism, where it facilitates the conversion of nutrients into ATP (adenosine triphosphate), the cell's energy currency. Its natural origin aligns with human biological pathways, enabling seamless integration into metabolic processes.
DL-6 8-thioctic acid: Often referred to as racemic alpha-lipoic acid, this synthetic mixture contains equal parts of the R-enantiomer and its mirror counterpart, the S-enantiomer (S-(-)-α-lipoic acid). The S-form does not occur naturally in humans and lacks the metabolic cofactor activity of the R-enantiomer. Traditional chemical synthesis produces this 50:50 blend, and separating the two enantiomers requires additional processing, which historically limited the availability of pure R-alpha-lipoic acid.
Advancements in biotechnological production, such as enzyme-catalyzed synthesis, have now enabled large-scale manufacturing of pure R-alpha-lipoic acid with high optical purity (≥98%), eliminating the need for enantiomeric separation and reducing production waste. This technological shift has further highlighted the limitations of the racemic mixture.

Bioavailability and Metabolic Integration
Bioavailability, the degree to which a compound is absorbed and utilized by the body, varies dramatically between the two forms, largely due to the presence of the inactive S-enantiomer in DL-6 8-thioctic acid.
Oral administration of alpha-lipoic acid faces inherent challenges: poor solubility, gastric instability, and hepatic degradation, resulting in an overall bioavailability of approximately 30% for conventional solid formulations. However, pure R-alpha-lipoic acid, particularly in optimized liquid formulations, circumvents these barriers. A 2014 study published in PubMed found that liquid R-alpha-lipoic acid achieved plasma concentrations comparable to intravenous administration, with accelerated absorption and prolonged stability, outperforming both solid R-alpha-lipoic acid formulations and racemic mixtures.
The S-enantiomer in DL-6 8-thioctic acid not only contributes no meaningful biological activity but may also interfere with the metabolism of the R-form. In insulin-resistant skeletal muscle of obese Zucker rats, acute administration of R-alpha-lipoic acid increased insulin-mediated glucose uptake by 64%, while the S-enantiomer had no effect. Chronic treatment with the S-form even exacerbated hyperinsulinemia, raising plasma insulin levels by 15% compared to controls, a counterproductive effect for individuals seeking metabolic support.
These findings underscore a key principle: the racemic mixture's bioavailability is not just "half as effective" as pure R-alpha-lipoic acid. The S-enantiomer acts as an inert (and potentially disruptive) diluent, reducing the proportional absorption and activity of the therapeutic R-form.

Clinical Efficacy: From Antioxidant Protection to Metabolic Support
The therapeutic gap between DL-6 8-thioctic acid and R-alpha-lipoic acid is most evident in clinical applications, where the R-enantiomer's natural compatibility with human biology translates to superior outcomes.
Antioxidant Activity
Alpha-lipoic acid is often called the "universal antioxidant" for its ability to scavenge free radicals, regenerate other antioxidants (vitamin C, vitamin E, glutathione), and chelate pro-oxidative metal ions. However, only the R-enantiomer can be efficiently reduced to dihydrolipoic acid (DHLA)—its more potent antioxidant metabolite—in human cells. DHLA scavenges peroxynitrite and lipid peroxyl radicals 2.2 times more effectively than the oxidized form (ALA) and protects DNA from oxidative damage by neutralizing twice as many radicals. Since the S-enantiomer cannot be converted to DHLA efficiently, DL-6 8-thioctic acid's overall antioxidant capacity is significantly diminished.
Diabetic Neuropathy
The most well-documented clinical use of alpha-lipoic acid is in treating diabetic peripheral neuropathy (DPN), a complication of diabetes characterized by nerve damage and sensory impairment. A meta-analysis of 47 clinical studies confirmed that alpha-lipoic acid improves nerve conduction velocity and reduces neuropathic pain, but subgroup analyses reveal that pure R-alpha-lipoic acid drives these benefits. In animal models of DPN, liquid R-alpha-lipoic acid accelerated the recovery of sensory and motor nerve function more rapidly than solid formulations or racemic mixtures, critical for patients managing chronic nerve pain.
Metabolic Health
For individuals with insulin resistance, prediabetes, or type 2 diabetes, R-alpha-lipoic acid offers targeted metabolic support. A 2021 meta-analysis of 28 clinical studies found that alpha-lipoic acid improves insulin sensitivity (HOMA-IR) in a dose- and time-dependent manner, but only when the supplement contains high proportions of the R-enantiomer. In contrast, DL-6 8-thioctic acid's variable efficacy is likely due to the S-enantiomer's interference with insulin signaling.
Other emerging applications, from reducing cardiovascular risk markers (e.g., Lp-PLA2, ox-LDL) in diabetics to improving endothelial function, also rely on pure R-alpha-lipoic acid. A 2020 randomized trial found that 1200mg/day of R-alpha-lipoic acid reduced cardiovascular risk factors in type 2 diabetics, while no such effect was observed with the racemic mixture.
Clinical Efficacy: From Antioxidant Protection to Metabolic Support
The therapeutic gap between DL-6 8-thioctic acid and R-alpha-lipoic acid is most evident in clinical applications, where the R-enantiomer's natural compatibility with human biology translates to superior outcomes.
Antioxidant Activity
Alpha-lipoic acid is often called the "universal antioxidant" for its ability to scavenge free radicals, regenerate other antioxidants (vitamin C, vitamin E, glutathione), and chelate pro-oxidative metal ions. However, only the R-enantiomer can be efficiently reduced to dihydrolipoic acid (DHLA)—its more potent antioxidant metabolite—in human cells. DHLA scavenges peroxynitrite and lipid peroxyl radicals 2.2 times more effectively than the oxidized form (ALA) and protects DNA from oxidative damage by neutralizing twice as many radicals. Since the S-enantiomer cannot be converted to DHLA efficiently, DL-6 8-thioctic acid's overall antioxidant capacity is significantly diminished.
Diabetic Neuropathy
The most well-documented clinical use of alpha-lipoic acid is in treating diabetic peripheral neuropathy (DPN), a complication of diabetes characterized by nerve damage and sensory impairment. A meta-analysis of 47 clinical studies confirmed that alpha-lipoic acid improves nerve conduction velocity and reduces neuropathic pain, but subgroup analyses reveal that pure R-alpha-lipoic acid drives these benefits. In animal models of DPN, liquid R-alpha-lipoic acid accelerated the recovery of sensory and motor nerve function more rapidly than solid formulations or racemic mixtures, critical for patients managing chronic nerve pain.
Metabolic Health
For individuals with insulin resistance, prediabetes, or type 2 diabetes, R-alpha-lipoic acid offers targeted metabolic support. A 2021 meta-analysis of 28 clinical studies found that alpha-lipoic acid improves insulin sensitivity (HOMA-IR) in a dose- and time-dependent manner, but only when the supplement contains high proportions of the R-enantiomer. In contrast, DL-6 8-thioctic acid's variable efficacy is likely due to the S-enantiomer's interference with insulin signaling.
Other emerging applications, from reducing cardiovascular risk markers (e.g., Lp-PLA2, ox-LDL) in diabetics to improving endothelial function, also rely on pure R-alpha-lipoic acid. A 2020 randomized trial found that 1200mg/day of R-alpha-lipoic acid reduced cardiovascular risk factors in type 2 diabetics, while no such effect was observed with the racemic mixture.
Clinical Efficacy: From Antioxidant Protection to Metabolic Support
The therapeutic gap between DL-6 8-thioctic acid and R-alpha-lipoic acid is most evident in clinical applications, where the R-enantiomer's natural compatibility with human biology translates to superior outcomes.
Antioxidant Activity
Alpha-lipoic acid is often called the "universal antioxidant" for its ability to scavenge free radicals, regenerate other antioxidants (vitamin C, vitamin E, glutathione), and chelate pro-oxidative metal ions. However, only the R-enantiomer can be efficiently reduced to dihydrolipoic acid (DHLA)—its more potent antioxidant metabolite—in human cells. DHLA scavenges peroxynitrite and lipid peroxyl radicals 2.2 times more effectively than the oxidized form (ALA) and protects DNA from oxidative damage by neutralizing twice as many radicals. Since the S-enantiomer cannot be converted to DHLA efficiently, DL-6 8-thioctic acid's overall antioxidant capacity is significantly diminished.
Diabetic Neuropathy
The most well-documented clinical use of alpha-lipoic acid is in treating diabetic peripheral neuropathy (DPN), a complication of diabetes characterized by nerve damage and sensory impairment. A meta-analysis of 47 clinical studies confirmed that alpha-lipoic acid improves nerve conduction velocity and reduces neuropathic pain, but subgroup analyses reveal that pure R-alpha-lipoic acid drives these benefits. In animal models of DPN, liquid R-alpha-lipoic acid accelerated the recovery of sensory and motor nerve function more rapidly than solid formulations or racemic mixtures, critical for patients managing chronic nerve pain.
Metabolic Health
For individuals with insulin resistance, prediabetes, or type 2 diabetes, R-alpha-lipoic acid offers targeted metabolic support. A 2021 meta-analysis of 28 clinical studies found that alpha-lipoic acid improves insulin sensitivity (HOMA-IR) in a dose- and time-dependent manner, but only when the supplement contains high proportions of the R-enantiomer. In contrast, DL-6 8-thioctic acid's variable efficacy is likely due to the S-enantiomer's interference with insulin signaling.
Other emerging applications, from reducing cardiovascular risk markers (e.g., Lp-PLA2, ox-LDL) in diabetics to improving endothelial function, also rely on pure R-alpha-lipoic acid. A 2020 randomized trial found that 1200mg/day of R-alpha-lipoic acid reduced cardiovascular risk factors in type 2 diabetics, while no such effect was observed with the racemic mixture.
Safety and Tolerability: A Question of Purity
Both forms of alpha-lipoic acid are generally considered safe at recommended doses, but DL-6 8-thioctic acid carries a slightly higher risk of mild adverse effects—likely due to impurities or the S-enantiomer itself.
Common side effects of alpha-lipoic acid include gastrointestinal discomfort (nausea, bloating) and, rarely, hypoglycemia in patients taking antidiabetic medications . However, a 2020 meta-analysis of 71 clinical trials (4,749 participants) found that pure R-alpha-lipoic acid had no significant adverse effects even at high doses (up to 1800mg/day). In contrast, some users of DL-6 8-thioctic acid report more frequent digestive upset, which may be attributed to the S-enantiomer’s accumulation in gastrointestinal tissues.
A rare but critical safety consideration is insulin autoimmune syndrome (IAS), a condition where antibodies attack insulin, leading to severe hypoglycemia. While cases are extremely uncommon, all reported instances of alpha-lipoic acid-associated IAS have involved racemic mixtures, suggesting the S-enantiomer may trigger immune responses in susceptible individuals.
Choosing Wisely: Practical Guidance for Consumers
For those seeking alpha-lipoic acid supplementation, the choice between DL-6 8-thioctic acid and R-alpha-lipoic acid is clear; pure R-alpha-lipoic acid offers superior efficacy, bioavailability, and safety. Here are key considerations:
Check the Label: Look for "R-alpha-lipoic acid," "(R)-(+)-α-lipoic acid," or "natural alpha-lipoic acid." Avoid products labeled simply "alpha-lipoic acid" or "DL-thioctic acid," as these are likely racemic mixtures.
Prioritize Purity: Opt for supplements with ≥98% R-enantiomer purity (HPLC-verified), as lower purity may indicate residual S-form.
Consider Formulation: Liquid or microencapsulated R-alpha-lipoic acid has higher bioavailability than solid tablets or capsules.
Dose Appropriately: For general antioxidant support, 200–600mg/day of pure R-alpha-lipoic acid is sufficient. For DPN or insulin resistance, 600–1200mg/day may be recommended under medical supervision.
The distinction between DL-6 8-thioctic acid and R-alpha-lipoic acid is not a trivial one; it is a difference between a synthetic mixture with limited utility and a natural compound optimized for human biology. From its role as a mitochondrial cofactor to its potent antioxidant and metabolic effects, R-alpha-lipoic acid's superiority is supported by decades of biochemical research and clinical evidence.
As the science of personalized nutrition advances, the era of "one-size-fits-all" supplements is fading. For anyone seeking to leverage alpha-lipoic acid's therapeutic potential, pure R-alpha-lipoic acid is the only choice that aligns with the principles of evidence-based care.
Choose Rebecca Bio-Tech
Rebecca Bio-Tech offers a premium solution for those seeking high-quality DL-6 8-thioctic acid, commonly known as Lipoic acid powder. This compound, scientifically referred to as 1,2-Dithiolane-3-pentanoic acid, is available in a specification of 99% purity, ensuring reliable performance for diverse applications.
With a molecular formula of C8H14O2S2 and a CAS number of 1077-28-7, this lipoic acid powder is ideal for researchers, pharmaceutical companies, and supplement manufacturers who demand precision and quality. At Rebecca Bio-Tech, we are committed to delivering superior products at competitive prices, backed by strict manufacturing standards.
For more information, to request samples, or to discuss custom requirements, please contact us at information@sxrebecca.com.
References
Packer, L., Witt, E. H., & Tritschler, H. J. (1995). Alpha-lipoic acid as a biological antioxidant. Free Radical Biology and Medicine, 19(2), 227–250.
Chemsrc. (n.d.). Lipoic acid (R-(+)-α-lipoic acid).Shurl.cc. (n.d.). Comparison of antioxidant effectiveness of lipoic acid and dihydrolipoic acid.
ChemicalBook. (2025).
PubMed. (2014). R-α-lipoic acid oral liquid formulation: pharmacokinetic parameters and therapeutic efficacy.
PubMed. (1997). Differential effects of lipoic acid stereoisomers on glucose metabolism.








